drug delivery
Teaming Magnetic Bacteria with Nanoparticles for Better Drug Delivery
Posted on by Dr. Francis Collins
Nanoparticles hold great promise for delivering next-generation therapeutics, including those based on CRISPR gene editing tools. The challenge is how to guide these tiny particles through the bloodstream and into the right target tissues. Now, scientists are enlisting some surprising partners in this quest: magnetic bacteria!
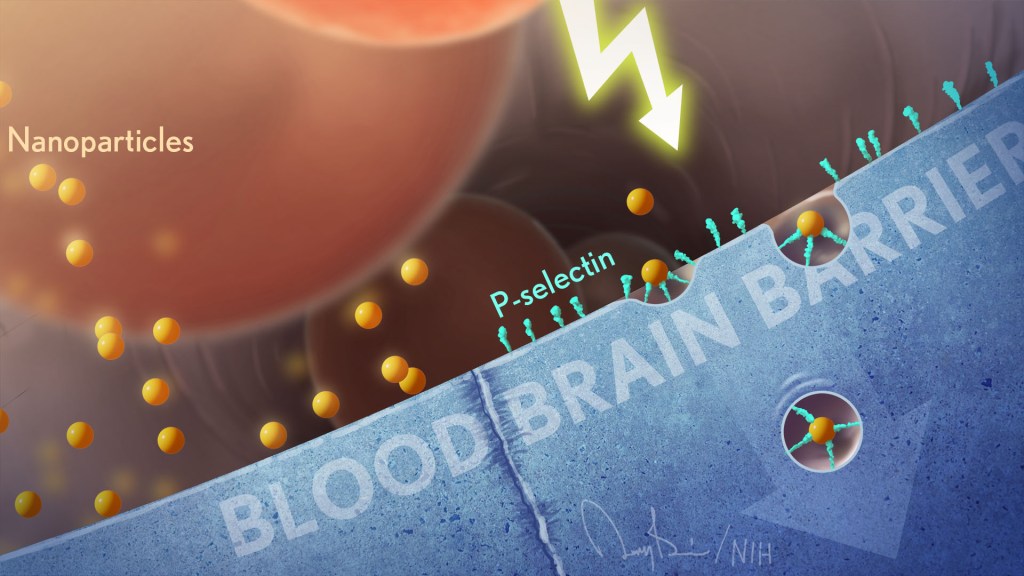
First a bit of background. Discovered in the 1960s during studies of bog sediments, “magnetotactic” bacteria contain magnetic, iron-rich particles that enable them to orient themselves to the Earth’s magnetic fields. To explore the potential of these microbes for targeted delivery of nanoparticles, the NIH-funded researchers devised the ingenious system you see in this fluorescence microscopy video. This system features a model blood vessel filled with a liquid that contains both fluorescently-tagged nanoparticles (red) and large swarms of a type of magnetic bacteria called Magnetospirillum magneticum (not visible).
At the touch of a button that rotates external magnetic fields, researchers can wirelessly control the direction in which the bacteria move through the liquid—up, down, left, right, and even “freestyle.” And—get this—the flow created by the synchronized swimming of all these bacteria pushes along any nearby nanoparticles in the same direction, even without any physical contact between the two. In fact, the researchers have found that this bacteria-guided system delivers nanoparticles into target model tissues three times faster than a similar system lacking such bacteria.
How did anyone ever dream this up? Most previous attempts to get nanoparticle-based therapies into diseased tissues have relied on simple diffusion or molecular targeting methods. Because those approaches are not always ideal, NIH-funded researchers Sangeeta Bhatia, Massachusetts Institute of Technology, Cambridge, MA, and Simone Schürle, formerly of MIT and now ETH Zurich, asked themselves: Could magnetic forces be used to propel nanoparticles through the bloodstream?
As a graduate student at ETH Zurich, Schürle had worked to develop and study tiny magnetic robots, each about the size of a cell. Those microbots, called artificial bacterial flagella (ABF), were designed to replicate the movements of bacteria, relying on miniature flagellum-like propellers to move them along in corkscrew-like fashion.
In a study published recently in Science Advances, the researchers found that the miniature robots worked as hoped in tests within a model blood vessel [1]. Using magnets to propel a single microbot, the researchers found that 200-nanometer-sized polystyrene balls penetrated twice as far into a model tissue as they did without the aid of the magnet-driven forces.
At the same time, others in the Bhatia lab were developing bacteria that could be used to deliver cancer-fighting drugs. Schürle and Bhatia wished they could direct those microbial swarms using magnets as they could with the microbots. That’s when they learned about the potential of M. magneticum and developed the experimental system demonstrated in the video above.
The researchers’ next step will be to test their magnetic approach to drug delivery in a mouse model. Ultimately, they think their innovative strategy holds promise for delivering nanoparticles carrying a wide range of therapeutic payloads right to a tumor, infection, or other diseased tissue. It’s yet another example of how basic research combined with outside-the-box thinking can lead to surprisingly creative solutions with real potential to improve human health.
References:
[1] Synthetic and living micropropellers for convection-enhanced nanoparticle transport. Schürle S, Soleimany AP, Yeh T, Anand GM, Häberli M, Fleming HE, Mirkhani N, Qiu F, Hauert S, Wang X, Nelson BJ, Bhatia SN. Sci Adv. 2019 Apr 26;5(4):eaav4803.
Links:
Nanotechnology (NIH)
What are genome editing and CRISPR-Cas9? (National Library of Medicine/NIH)
Sangeeta Bhatia (Massachusetts Institute of Technology, Cambridge, MA)
Simone Schürle-Finke (ETH Zurich, Switzerland)
NIH Support: National Cancer Institute; National Institute of General Medical Sciences
Building Nanoparticles for Kidney Disease
Posted on by Dr. Francis Collins

Great things sometimes come in small packages. That’s certainly true in the lab of Eun Ji Chung at the University of Southern California, Los Angeles. Chung and her team each day wrap their brains around bioengineering 3-D nanoparticles, molecular constructs that measure just a few billionths of a meter.
Chung recently received an NIH Director’s 2018 New Innovator Award to bring the precision of nanomedicine to autosomal dominant polycystic kidney disease (ADPKD), a relatively common inherited disorder that affects about 600,000 Americans and 12 million people worldwide.
By age 60, about half of those battling ADPKD will have kidney failure, requiring dialysis or a kidney transplant to stay alive. For people with ADPKD, a dominantly inherited gene mutation causes clusters of fluid-filled cysts to form in the kidneys that grow larger over time. The cysts can grow very large and displace normal kidney tissue, progressively impairing function.
For Chung, the goal is to design nanoparticles of the right size and configuration to deliver therapeutics to the kidneys in safe, effective amounts. Our kidneys constantly filter blood, clearing out wastes that are removed via urine. So, Chung and her team will exploit the fact that most molecules in the bloodstream measuring less than 10 nanometers in diameter enter the kidneys, where they are gradually processed and eliminated from the body. This process will give nanoparticles time to bind there and release any therapeutic molecules they may be carrying directly to the cysts that cluster on the kidneys of people with ADPKD.
Chung’s research couldn’t be more timely. Though ADPKD isn’t curable right now, the Food and Drug Administration (FDA) last year approved Jynarque™ (tolvaptan), the first treatment in the United States to slow the decline in kidney function in ADPKD patients, based on tests of the rate of kidney filtration. Other approved drugs, such as metformin and rapamycin, have shown potential for repurposing to treat people with ADPKD. So, getting these and other potentially life-saving drugs directly to the kidneys, while minimizing the risk of serious side effects in the liver and elsewhere in the body, will be key.
Most FDA-approved nanoparticle therapies are administered intravenously, often for treatment of cancer. Because ADPKD is chronic and treatment can last for decades, Chung wants to develop an easy-to-take pill to get these nanoparticles into the kidneys.
But oral administration raises its own set of difficulties. The nanoparticles must get from the stomach and the rest of the gastrointestinal tract to the bloodstream. And then if nanoparticles exceed 10 nanometers in diameter, the body typically routes them to the liver for clearance, rather than the kidneys.
While Chung brainstorms strategies for oral administration, she’s also considering developing a smart bandage to allow the nanoparticles to pass readily through the skin and, eventually, into the bloodstream. It would be something similar to the wearable skin patch already featured on the blog.
In the meantime, Chung continues to optimize the size, shape, and surface charge of her nanoparticles. Right now, they have components to target the kidneys, provide a visual signal for tracking, enhance the nanoparticle’s lifespan, and carry a therapeutic molecule. Because positively charged molecules are preferentially attracted to the kidney, Chung has also spent untold hours adjusting the charge on her nanoparticles.
But through all the hard work, Chung and her team continue to prove that great things may one day come in very small packages. And that could ultimately prove to be a long-awaited gift for the millions of people living with ADPKD.
Links:
Polycystic Kidney Disease (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Video: Faculty Profile – Eun Ji Chung (University of Southern California, Los Angeles)
Chung Laboratory (USC)
Chung Project Information (NIH RePORTER)
NIH Director’s New Innovator Award (Common Fund)
NIH Support: Common Fund; National Institute of Diabetes and Digestive and Kidney Diseases
Tracking Peptides in Cell Soup
Posted on by Dr. Francis Collins

Credit: William Wimley, Tulane University, New Orleans
If you think this soup looks unappealing for this year’s Thanksgiving feast, you’re right! If you were crazy enough to take a sip, you’d find it to be virtually flavorless—just a salty base (red) with greasy lipid globules (green) floating on top. But what this colorful concoction lacks in taste, it makes up for as a valuable screening tool for peptides, miniature versions of proteins that our bodies use to control many cellular processes.
In this image, William Wimley, an NIH-supported researcher at Tulane University, New Orleans, has stirred up the soup and will soon add some peptides. These peptides aren’t made by our cells, though. They’re synthesized in the lab, allowing Wimley and team to tweak their chemical structures and hopefully create ones with therapeutic potential, particularly as smart-delivery systems to target cells with greater precision and deliver biological cargoes such as drugs [1].
Seven More Awesome Technologies Made Possible by Your Tax Dollars
Posted on by Dr. Francis Collins
We live in a world energized by technological advances, from that new app on your smartphone to drones and self-driving cars. As you can see from this video, NIH-supported researchers are also major contributors, developing a wide range of amazing biomedical technologies that offer tremendous potential to improve our health.
Produced by the NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB), this video starts by showcasing some cool fluorescent markers that are custom-designed to light up specific cells in the body. This technology is already helping surgeons see and remove tumor cells with greater precision in people with head and neck cancer [1]. Further down the road, it might also be used to light up nerves, which can be very difficult to see—and spare—during operations for cancer and other conditions.
Other great things to come include:
- A wearable tattoo that detects alcohol levels in perspiration and wirelessly transmits the information to a smartphone.
- Flexible coils that produce high quality images during magnetic resonance imaging (MRI) [2-3]. In the future, these individualized, screen-printed coils may improve the comfort and decrease the scan times of people undergoing MRI, especially infants and small children.
- A time-release capsule filled with a star-shaped polymer containing the anti-malarial drug ivermectin. The capsule slowly dissolves in the stomach over two weeks, with the goal of reducing the need for daily doses of ivermectin to prevent malaria infections in at-risk people [4].
- A new radiotracer to detect prostate cancer that has spread to other parts of the body. Early clinical trial results show the radiotracer, made up of carrier molecules bonded tightly to a radioactive atom, appears to be safe and effective [5].
- A new supercooling technique that promises to extend the time that organs donated for transplantation can remain viable outside the body [6-7]. For example, current technology can preserve donated livers outside the body for just 24 hours. In animal studies, this new technique quadruples that storage time to up to four days.
- A wearable skin patch with dissolvable microneedles capable of effectively delivering an influenza vaccine. This painless technology, which has produced promising early results in humans, may offer a simple, affordable alternative to needle-and-syringe immunization [8].
If you like what you see here, be sure to check out this previous NIH video that shows six more awesome biomedical technologies that your tax dollars are helping to create. So, let me extend a big thanks to you from those of us at NIH—and from all Americans who care about the future of their health—for your strong, continued support!
References:
[1] Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. Wiley Interdiscip Rev Syst Biol Med. 2018 Feb 23.
[2] Screen-printed flexible MRI receive coils. Corea JR, Flynn AM, Lechêne B, Scott G, Reed GD, Shin PJ, Lustig M, Arias AC. Nat Commun. 2016 Mar 10;7:10839.
[3] Printed Receive Coils with High Acoustic Transparency for Magnetic Resonance Guided Focused Ultrasound. Corea J, Ye P, Seo D, Butts-Pauly K, Arias AC, Lustig M. Sci Rep. 2018 Feb 21;8(1):3392.
[4] Oral, ultra-long-lasting drug delivery: Application toward malaria elimination goals. Bellinger AM, Jafari M1, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee YL, Mazdiyasni H, Kogan L, Barman R, Cleveland C, Booth L, Bensel T, Minahan D, Hurowitz HM, Tai T, Daily J, Nikolic B, Wood L, Eckhoff PA, Langer R, Traverso G. Sci Transl Med. 2016 Nov 16;8(365):365ra157.
[5] Clinical Translation of a Dual Integrin avb3– and Gastrin-Releasing Peptide Receptor–Targeting PET Radiotracer, 68Ga-BBN-RGD. Zhang J, Niu G, Lang L, Li F, Fan X, Yan X, Yao S, Yan W, Huo L, Chen L, Li Z, Zhu Z, Chen X. J Nucl Med. 2017 Feb;58(2):228-234.
[6] Supercooling enables long-term transplantation survival following 4 days of liver preservation. Berendsen TA, Bruinsma BG, Puts CF, Saeidi N, Usta OB, Uygun BE, Izamis ML, Toner M, Yarmush ML, Uygun K. Nat Med. 2014 Jul;20(7):790-793.
[7] The promise of organ and tissue preservation to transform medicine. Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, et a. Nat Biotechnol. 2017 Jun 7;35(6):530-542.
[8] The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR; TIV-MNP 2015 Study Group.
Links:
National Institute of Biomedical Imaging and Bioengineering (NIH)
Center for Wearable Sensors (University of California, San Diego)
Hyperpolarized MRI Technology Resource Center (University of California, San Francisco)
Center for Engineering in Medicine (Massachusetts General Hospital, Boston)
Center for Drug Design, Development and Delivery (Georgia Tech University, Atlanta)
NIH Support: National Institute of Biomedical Imaging and Bioengineering; National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of Allergy and Infectious Diseases
Creative Minds: Giving Bacteria Needles to Fight Intestinal Disease
Posted on by Dr. Francis Collins
For Salmonella and many other disease-causing bacteria that find their way into our bodies, infection begins with a poke. That’s because these bad bugs are equipped with a needle-like protein filament that punctures the outer membrane of human cells and then, like a syringe, injects dozens of toxic proteins that help them replicate.
Cammie Lesser at Massachusetts General Hospital and Harvard Medical School, Cambridge, and her colleagues are now on a mission to bioengineer strains of bacteria that don’t cause disease to make these same syringes, called type III secretion systems. The goal is to use such “good” bacteria to deliver therapeutic molecules, rather than toxins, to human cells. Their first target is the gastrointestinal tract, where they hope to knock out hard-to-beat bacterial infections or to relieve the chronic inflammation that comes with inflammatory bowel disease (IBD).
Snapshots of Life: Biological Bubble Machine
Posted on by Dr. Francis Collins
As kids, most of us got a bang out of blowing soap bubbles and watching them float around. Biologists have learned that some of our cells do that too. On the right, you can see two cells (greenish yellow) in the process of forming bubbles, or plasma membrane vesicles (PMVs). During this blebbing process, a cell’s membrane temporarily disassociates from its underlying cytoskeleton, forming a tiny pouch that, over the course of about 30 minutes, is “inflated” with a mix of proteins and lipids from inside the cell. After the PMVs are fully filled, these bubble-like structures are pinched off and released, like those that you see in the background. Certain cells constantly release PMVs, along with other types of vesicles, and may use those to communicate with other cells throughout the body.
This particular image, an entrant in the Biophysical Society’s 2017 Art of Science Image Contest, was produced by researchers working in the NIH-supported lab of Jeanne Stachowiak at the University of Texas at Austin. Stachowiak’s group is among the first to explore the potential of PMVs as specialized drug-delivery systems to target cancer and other disorders [1].
Until recently, most efforts to exploit vesicles for therapeutic uses have employed synthetic versions of a different type of vesicle, called an exosome. But Stachowiak and others have realized that PMVs come with certain built-in advantages. A major one is that a patient’s own cells could in theory serve as the production facility.
Creative Minds: Can Diseased Cells Help to Make Their Own Drugs?
Posted on by Dr. Francis Collins

Matthew Disney grew up in a large family in Baltimore in the 1980s. While his mother worked nights, Disney and his younger brother often tagged along with their father in these pre-Internet days on calls to fix the microfilm machines used to view important records at hospitals, banks, and other places of business. Watching his father take apart the machines made Disney want to work with his hands one day. Seeing his father work tirelessly for the sake of his family also made him want to help others.
Disney found a profession that satisfied both requirements when he fell in love with chemistry as an undergraduate at the University of Maryland, College Park. Now a chemistry professor at The Scripps Research Institute, Jupiter, FL, Disney is applying his hands and brains to develop a treatment strategy that aims to control the progression of a long list of devastating disorders that includes Huntington’s disease, amyotrophic lateral sclerosis (ALS), and various forms of muscular dystrophy.
The 30 or so health conditions on Disney’s list have something in common. They are caused by genetic glitches in which repetitive DNA letters (CAGCAGCAG, for example) in transcribed regions of the genome cause some of the body’s cells and tissues to produce unwieldy messenger RNA molecules that interfere with normal cellular activities, either by binding other intracellular components or serving as templates for the production of toxic proteins.
The diseases on Disney’s list also have often been considered “undruggable,” in part because the compounds capable of disabling the lengthy, disease-causing RNA molecules are generally too large to cross cell membranes. Disney has found an ingenious way around that problem [1]. Instead of delivering the finished drug, he delivers smaller building blocks. He then uses the cell and its own machinery, including the very aberrant RNA molecules he aims to target, as his drug factory to produce those larger compounds.
Disney has received an NIH Director’s 2015 Pioneer Award to develop this innovative drug-delivery strategy further. He will apply his investigational approach initially to treat a common form of muscular dystrophy, first using human cells in culture and then in animal models. Once he gets that working well, he’ll move on to other conditions including ALS.
What’s appealing about Disney’s approach is that it makes it possible to treat disease-affected cells without affecting healthy cells. That’s because his drugs can only be assembled into their active forms in cells after they are templated by those aberrant RNA molecules.
Interestingly, Disney never intended to study human diseases. His lab was set up to study the structure and function of RNA molecules and their interactions with other small molecules. In the process, he stumbled across a small molecule that targets an RNA implicated in a rare form of muscular dystrophy. His niece also has a rare incurable disease, and Disney saw a chance to make a difference for others like her. It’s a healthy reminder that the pursuit of basic scientific questions often can lead to new and unexpectedly important medical discoveries that have the potential to touch the lives of many.
Reference:
[1] A toxic RNA catalyzes the in cellulo synthesis of its own inhibitor. Rzuczek SG, Park H, Disney MD. Angew Chem Int Ed Engl. 2014 Oct 6;53(41):10956-10959.
Links:
Disney Lab (The Scripps Research Institute, Jupiter, FL)
Disney NIH Project Information (NIH RePORTER)
NIH Director’s Pioneer Award Program
NIH Support: Common Fund; National Institute of Neurological Disorders and Stroke
Next Page