fat
Saving Fat for Lean Times
Posted on by Lawrence Tabak, D.D.S., Ph.D.
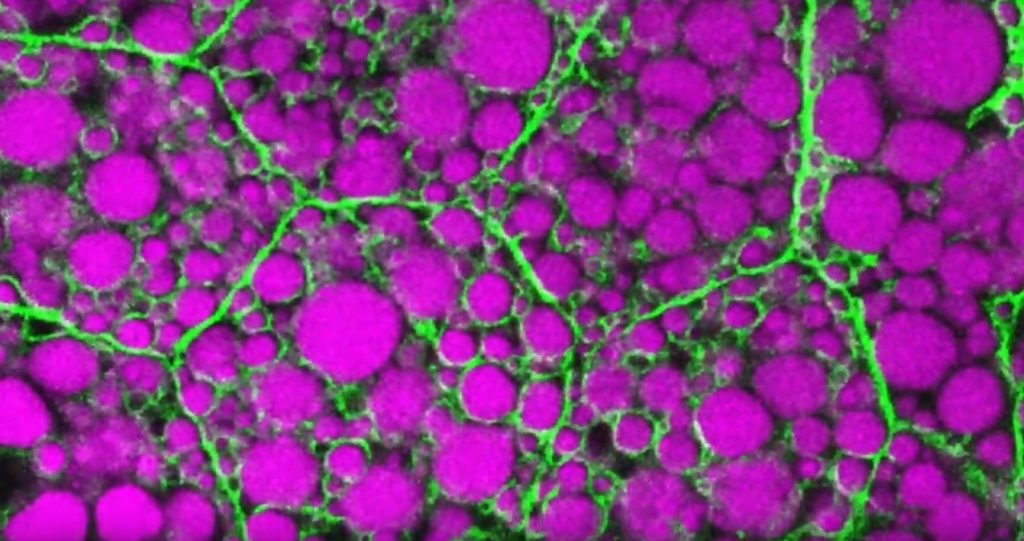
Humans and all multi-celled organisms, or metazoans, have evolved through millennia into a variety of competing shapes, sizes, and survival strategies. But all metazoans still share lots of intriguing cell biology, including the ability to store excess calories as fat. In fact, many researchers now consider fat-storing cells to be “nutrient sinks,” or good places for the body to stash excess sugars and lipids. Not only can these provide energy needed to survive a future famine, this is a good way to sequester extra molecules that could prove toxic to cells and organs.
Here’s something to think about the next time you skip a meal. Fat-storing cells organize their fat reserves spatially, grouping them into specific pools of lipid types, in order to generate needed energy when food is scarce.
That’s the story behind this striking image taken in a larval fruit fly (Drosophila melanogaster). The image captures fat-storing adipocytes in an organ called a fat body, where a larval fruit fly stores extra nutrients. It’s like the fat tissue in mammals. You can see both large and small lipid droplets (magenta) inside polygon-shaped fat cells, or adipocytes, lined by their plasma membranes (green). But notice that the small lipid droplets are more visibly lined by green, as only these are destined to be saved for later and exported when needed into the fly’s bloodstream.
Working in Mike Henne’s lab at the University of Texas Southwestern Medical Center, Dallas, research associate Rupali Ugrankar discovered how this clever fat-management system works in Drosophila [1]. After either feeding flies high-or-extremely low-calorie diets, Ugrankar used a combination of high-resolution fluorescence confocal microscopy and thin-section transmission electron microscopy to provide a three-dimensional view of adipocytes and their lipid droplets inside.
She observed two distinct sizes of lipid droplets and saw that only the small ones clustered at the cell surface membrane. The adipocytes contorted their membrane inward to grab these small droplets and package them into readily exportable energy bundles.
Ugrankar saw that during times of plenty, a protein machine could fill these small membrane-wrapped fat droplets with lots of triacylglycerol, a high-energy, durable form of fat storage. Their ready access at the surface of the adipocyte allows the fly to balance lipid storage locally with energy release into its blood in times of famine.
Ugrankar’s adeptness at the microscope resulted in this beautiful photo, which was earlier featured in the American Society for Cell Biology’s Green Fluorescent Protein Image and Video Contest. But her work and that of many others help to open a vital window into nutrition science and many critical mechanistic questions about the causes of obesity, insulin resistance, hyperglycemia, and even reduced lifespan.
Such basic research will provide the basis for better therapies to correct these nutrition-related health problems. But the value of basic science must not be forgotten—some of the most important leads could come from a tiny insect in its larval state that shares many aspects of mammalian metabolism.
Reference:
[1] Drosophila Snazarus regulates a lipid droplet population at plasma membrane-droplet contacts in adipocytes. Ugrankar R, Bowerman J, Hariri H, Chandra M, et al. Dev Cell. 2019 Sep 9;50(5):557-572.e5.
Links:
The Interactive Fly (Society for Developmental Biology, Rockville, MD)
Henne Lab (University of Texas Southwestern Medical Center, Dallas)
NIH Support: National Institute of General Medical Sciences
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Snapshots of Life
Tags: 2019 Green Fluorescent Protein Image and Video Contest, adipocyte, American Society for Cell Biology, basic research, cell biology, Drosophila melanogaster, fat, fat body, fat cells, fat storage, fluorescence microscopy, fruit fly, hyperglycemia, imaging, insulin resistance, lifespan, lipid droplet, lipid storage, lipids, metabolism, model organisms, nutrient sink, obesity, triacylglycerol
Large Study Reveals Prevalence, Health Benefits of Brown Fat
Posted on by Dr. Francis Collins
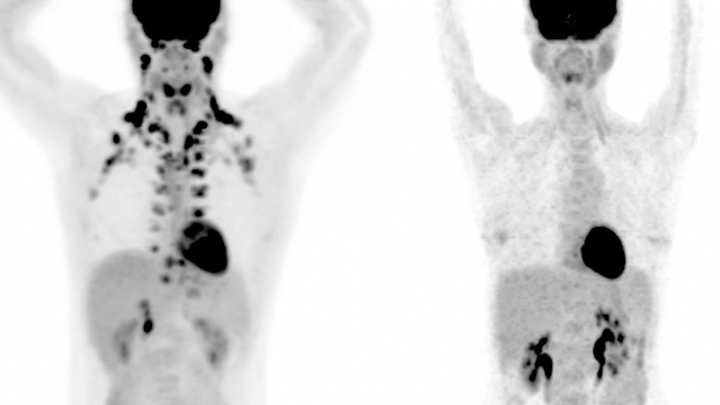
It’s pretty easy to spot differences between the two people on these positron emission tomography (PET) scans. In the scan of the male individual on the left, you see lots of small, dark spots around the neck and shoulders. But you can’t see any on the female on the right. What’s the explanation? Is this a sex difference? No! Brown fat!
This energy-burning type of fat happens to show up as small, dark spots in the neck and shoulder area on PET scan studies. So, as these scans reveal, the individual on the left possesses an abundance of brown fat, while the person on the right has essentially none. This wide range of difference in abundance is true for both men and women.
Researchers’ interest in brown fat began to heat up (sorry about that!) more than a decade ago when it was discovered that certain adults have persistently high levels of brown fat. It’s long been known that babies have brown fat, but it had been thought this fat generally vanished as children grew up. It turns out that adults who hold onto their brown fat are less likely to be overweight than adults who do not. That’s because brown fat actually burns extra calories, instead of storing it in the way the more familiar white fat does.
But are people with more brown fat actually any healthier? After studying about 130,000 PET scans from more than 52,000 people, researchers led by Paul Cohen, The Rockefeller University Hospital, New York, NY, say that the answer is “yes” in certain key areas. In a recent study in the journal Nature Medicine, they found that people with detectable brown fat had a lower incidence of many cardiovascular and metabolic conditions, including type 2 diabetes, congestive heart failure, and high blood pressure.
Studies to explore the health benefits of brown fat have been challenging to do. That’s because brown fat only shows up on PET scans, which measure how much glucose various tissues consume, an indication of their metabolic activity. What’s more, PET scans are quite costly and involve radiation exposure. So, researchers have been reluctant to ask healthy people to undergo a PET scan just to look at brown fat. But a solution occurred to the study’s first author Tobias Becher, who was aware that thousands of patients at nearby Memorial Sloan Kettering Cancer Center were undergoing PET scans each year as part of routine evaluation and care. In fact, cancer doctors often make note of brown fat on PET scans, if only to make sure it’s not mistaken for cancer.
So, the Cohen lab teamed up with Memorial Sloan Kettering Cancer Center radiologists Heiko Schöder and Andreas G. Wibmer to review many thousands of PET scans for the presence of brown fat. And they found it in about one of 10 people.
Next, they looked for health differences between the 10 percent of people with brown fat and the 90 percent who lack it. The differences turned out be striking. Type 2 diabetes was about half as prevalent in folks with detectable brown fat compared to those without. Individuals with brown fat also were less likely to have high cholesterol, high blood pressure, congestive heart failure, and coronary artery disease.
The findings suggest that brown fat may even help to offset the negative health effects of obesity. The researchers found that obese people with brown fat had a health profile that otherwise appeared more similar to individuals who weren’t obese. In fact, the benefits of brown fat were more pronounced in individuals who were overweight or obese than they were in people of normal weight.
Still, the researchers note that people with cancer might tend to show differences in brown fat compared to healthy adults. There’s some evidence also that prevalence may vary across cancer types and stages. The researchers took those variables into account in their studies. It’s also known that women are more likely to have brown fat than men and that the amount of brown fat tends to decline with age. What’s not yet well understood is whether differences in brown fat exist among people of different racial and ethnic backgrounds, and whether specific genetic factors are involved.
So, plenty of questions remain! Researchers not only want to figure out why some adults have so much more brown fat than others, they want to explore whether brown fat produces hormones that may add to its calorie-burning benefits. The hope is that these and other discoveries could eventually lead to new strategies for treating obesity, diabetes, and other metabolic conditions.
Reference:
[1] Brown adipose tissue is associated with cardiometabolic health. Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, Butler SD, Jiang CS, Vaughan R, Schöder H, Mark A, Cohen P. Nat Med. 2021 Jan;27(1):58-65.
Links:
Paul Cohen (The Rockefeller University, New York, NY)
Heiko Schöder (Memorial Sloan Kettering Cancer Center, NY)
Andreas Wibmer (Memorial Sloan Kettering Cancer Center, NY)
NIH Support: National Center for Advancing Translational Sciences
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Why When You Eat Might Be as Important as What You Eat
Posted on by Dr. Francis Collins

About 1 in 3 American adults have metabolic syndrome, a group of early warning signs for increased risk of type 2 diabetes, heart disease, and stroke. To help avoid such health problems, these folks are often advised to pay close attention to the amount and type of foods they eat. And now it seems there may be something else to watch: how food intake is spaced over a 24-hour period.
In a three-month pilot study, NIH-funded researchers found that when individuals with metabolic syndrome consumed all of their usual daily diet within 10 hours—rather than a more customary span of about 14 hours—their early warning signs improved. Not only was a longer stretch of daily fasting associated with moderate weight loss, in some cases, it was also tied to lower blood pressure, lower blood glucose levels, and other improvements in metabolic syndrome.
The study, published in Cell Metabolism, is the result of a joint effort by Satchidananda Panda, Salk Institute for Biological Sciences, La Jolla, CA, and Pam R. Taub, University of California, San Diego [1]. It was inspired by Panda’s earlier mouse studies involving an emerging dietary intervention, called time-restricted eating (TRE), which attempts to establish a consistent daily cycle of feeding and fasting to create more stable rhythms for the body’s own biological clock [2, 3].
But would observations in mice hold true for humans? To find out, Panda joined forces with Taub, a cardiologist and physician-scientist. The researchers enlisted 19 men and women with metabolic syndrome, defined as having three or more of five specific risk factors: high fasting blood glucose, high blood pressure, high triglyceride levels, low “good” cholesterol, and/or extra abdominal fat. Most participants were obese and taking at least one medication to help manage their metabolic risk factors.
In the study, participants followed one rule: eat anything that you want, just do so over a 10-hour period of your own choosing. So, for the next three months, these folks logged their eating times and tracked their sleep using a special phone app created by the research team. They also wore activity and glucose monitors.
By the pilot study’s end, participants following the 10-hour limitation had lost on average 3 percent of their weight and about 3 percent of their abdominal fat. They also lowered their cholesterol and blood pressure. Although this study did not find 10-hour TRE significantly reduced blood glucose levels in all participants, those with elevated fasting blood glucose did have improvement. In addition, participants reported other lifestyle improvements, including better sleep.
The participants generally saw their metabolic health improve without skipping meals. Most chose to delay breakfast, waiting about two hours after they got up in the morning. They also ate dinner earlier, about three hours before going to bed—and then did no late night snacking.
After the study, more than two-thirds reported that they stuck with the 10-hour eating plan at least part-time for up to a year. Some participants were able to cut back or stop taking cholesterol and/or blood-pressure-lowering medications.
Following up on the findings of this small study, Taub will launch a larger NIH-supported clinical trial involving 100 people with metabolic syndrome. Panda is now exploring in greater detail the underlying biology of the metabolic benefits observed in the mice following TRE.
For people looking to improve their metabolic health, it’s a good idea to consult with a doctor before making significant changes to one’s eating habits. But the initial data from this study indicate that, in addition to exercising and limiting portion size, it might also pay to watch the clock.
References:
[1] Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleisher JG, Panda S, Taub PR. Cell Metab. 2019 Jan 7; 31: 1-13. Epub 2019 Dec 5.
[2] Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Cell Metab. 2012 Jun 6;15(6):848-60.
[3] Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Chaix A, Zarrinpar A, Miu P, Panda S. Cell Metab. 2014 Dec 2;20(6):991-1005.
Links:
Metabolic Syndrome (National Heart, Lung, and Blood Institute/NIH)
Obesity (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Body Weight Planner (NIDDK/NIH)
Satchidananda Panda (Salk Institute for Biological Sciences, La Jolla, CA)
Taub Research Group (University of California, San Diego)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: bad cholesterol, biological clock, blood glucose, blood pressure, circadian rhythms, diet, fasting, fat, food, lipids, metabolic syndrome, metabolism, obesity, pilot study, sleep, time-restricted eating, TRE, triglycerides, weight loss
Americans Are Still Eating Too Much Added Sugar, Fat
Posted on by Dr. Francis Collins

Most of us know one of the best health moves we can make is to skip the junk food and eat a nutritious, well-balanced diet. But how are we doing at putting that knowledge into action? Not so great, according to a new analysis that reveals Americans continue to get more than 50 percent of their calories from low-quality carbohydrates and artery-clogging saturated fat.
In their analysis of the eating habits of nearly 44,000 adults over 16 years, NIH-funded researchers attributed much of our nation’s poor dietary showing to its ongoing love affair with heavily processed fast foods and snacks. But there were a few bright spots. The analysis also found that, compared to just a few decades ago, Americans are eating more foods with less added sugar, as well as more whole grains (e.g., brown rice, quinoa, rolled oats), plant proteins (e.g., nuts, beans), and sources of healthy fats (e.g., olive oil).
Over the last 20-plus years, research has generated new ideas about eating a proper diet. In the United States, the revised thinking led to the 2015-2020 Dietary Guidelines for Americans. They recommend eating more fruits, vegetables, whole grains, and other nutrient-dense foods, while limiting foods containing added sugars, saturated fats, and salt.
In the report published in JAMA, a team of researchers wanted to see how Americans are doing at following the new guidelines. The team was led by Shilpa Bhupathiraju, Harvard T. H. Chan School of Public Health, Boston, and Fang Fang Zhang, Tufts University, Boston.
To get the answer, the researchers looked to the National Health and Nutrition Examination Survey (NHANES). The survey includes a nationally representative sample of U.S. adults, age 20 or older, who had answered questions about their food and beverage intake over a 24-hour period at least once during nine annual survey cycles between 1999-2000 and 2015-2016.
The researchers assessed the overall quality of the American diet using the Healthy Eating Index-2015 (HEI-2015), which measures adherence to the 2015-2020 Dietary Guidelines. The HEI-2015 scores range from 0 to 100, with the latter number being a perfect, A-plus score. The analysis showed the American diet barely inching up over the last two decades from a final score of 55.7 to 57.7.
That, of course, is still far from a passing grade. Some of the common mistakes identified:
• Refined grains, starchy vegetables, and added sugars still account for 42 percent of the average American’s daily calories.
• Whole grains and fruits provide just 9 percent of daily calories.
• Saturated fat consumption remains above 10 percent of daily calories, as many Americans continue to eat more red and processed meat.
Looking on the bright side, the data do indicate more Americans are starting to lean toward the right choices. They are getting slightly more of their calories from healthier whole grains and a little less from added sugar. Americans are also now looking a little more to whole grains, nuts, and beans as a protein source. It’s important to note, though, these small gains weren’t seen in lower income groups or older adults.
The bottom line is most Americans still have an awfully long way to go to shape up their diets. The question is: how to get there? There are plenty of good choices that can help to turn things around, from reading food labels and limiting calories or portion sizes to exercising and finding healthy recipes that suit your palate.
Meanwhile, nutrition research is poised for a renaissance. Tremendous progress is being made in studying the microbial communities, or microbiomes, helping to digest our foods. The same is true for studies of energy metabolism, genetic variation influencing our dietary preferences, and the effects of aging.
This is an optimum time to enhance the science and evidence base for human nutrition. That may result in some updating of the scoring system for the nation’s dietary report card. But it will be up to all of us to figure out how to ace it.
References:
[1] Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999-2016. Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, Mozaffarian D, Zhang FF, Bhupathiraju SN. JAMA. 2019 Sep 24;322(12):1178-1187.
Links:
Eat Right (National Heart, Lung, and Blood Institute/NIH)
Dietary Fats (MedlinePlus, National Library of Medicine/NIH)
ChooseMyPlate (U.S. Department of Agriculture)
Healthy Eating Index (Department of Agriculture)
NIH Nutrition Research Task Force (National Institute of Diabetes and Digestive and Kidney Disease/NIH)
Dietary Guidelines for Americans (U.S. Department of Health and Human Services)
Shilpa Bhupathiraju (Harvard T. H. Chan School of Public Health, Boston)
Fang Fang Zhang (Tufts University, Boston)
NIH Support: National Institute on Minority Health and Health Disparities; National Institute of Diabetes and Digestive and Kidney Diseases
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: 2015-2020 Dietary Guidelines for Americans, aging, carbohydrates, diet, dietary guidelines, eating, energy metabolism, fat, food, fruits, Healthy Eating Index, high-fat diet, junk food, low-quality carbohydrates, microbiome, National Health and Nutrition Examination Survey, NHANES, nutrition, plant proteins, refined grains, salt, saturated fat, sugar, vegetables, whole grains
Ultra-Processed Diet Leads to Extra Calories, Weight Gain
Posted on by Dr. Francis Collins

If you’ve ever tried to lose a few pounds or just stay at a healthy weight, you’ve likely encountered a dizzying array of diets, each with passionate proponents: low carb, low fat, keto, paleo, vegan, Mediterranean, and so on. Yet most nutrition experts agree on one thing: it’s best to steer clear of ultra-processed foods. Now, there’s some solid scientific evidence to back up that advice.
In the first randomized, controlled study to compare the effects of ultra-processed with unprocessed foods, NIH researchers found healthy adults gained about a pound per week when they were given a daily diet high in ultra-processed foods, which often contain ingredients such as hydrogenated fats, high fructose corn syrup, flavoring agents, emulsifiers, and preservatives. In contrast, when those same people ate unprocessed whole foods, they lost weight.
Intriguingly, the weight differences on the two diets occurred even though both kinds of foods had been carefully matched from a nutritional standpoint, including calorie density, fiber, fat, sugar, and salt. For example, breakfast for the ultra-processed group might consist of a bagel with cream cheese and turkey bacon, while the unprocessed group might be offered oatmeal with bananas, walnuts, and skim milk.
The explanation for the differences appears to lie in the fact that study participants were free to eat as little or as much food as they wished at mealtimes and to snack between meals. It turns out that when folks were on the ultra-processed diet they ate significantly more—about 500 extra calories per day on average—than when they were on the unprocessed diet. And, as you probably know, more calories without more exercise usually leads to more weight!
This might not seem new to you. After all, it has been tempting for some time to suggest a connection between the rise of packaged, ultra-processed foods and America’s growing waistlines. But as plausible as it might seem that such foods may encourage overeating, perhaps because of their high salt, sugar, and fat content, correlation is not causation and controlled studies of what people actually eat are tough to do. As a result, definitive evidence directly tying ultra-processed foods to weight gain has been lacking.
To explore the possible connection in the study now reported in Cell Metabolism, researchers at NIH’s National Institute of Diabetes and Digestive and Kidney Diseases took advantage of the Metabolic Clinical Research Unit at the NIH Clinical Center, Bethesda, MD. The unit is specially equipped to study issues involving diet and metabolism.
The researchers asked 20 healthy men and women of stable weight to stay at the center for 28 days. Each volunteer was randomly assigned to eat either an ultra-processed or unprocessed diet for two consecutive weeks. At that point, they switched to the other diet for another two weeks.
Both diets consisted of three daily meals, and volunteers were given permission to eat as much food as they liked. Importantly, a team of dieticians had carefully designed the ultra-processed and unprocessed meals such that they were well matched for total calories, calorie density, macronutrients, fiber, sugars, and salt.
At lunch, for example, one of the study’s processed meals consisted of quesadillas, refried beans, and diet lemonade. An unprocessed lunch consisted of a spinach salad with chicken breast, apple slices, bulgur, and sunflower seeds with a side of grapes.
The main difference between each diet was the proportion of calories derived from ultra-processed versus unprocessed foods as defined by the NOVA diet classification system. This system categorizes food based on the nature, extent, and purpose of food processing, rather than its nutrient content.
Each week, researchers measured the energy expenditure, weight, and changes in body composition of all volunteers. After two weeks on the ultra-processed diet, volunteers gained about two pounds on average. That’s compared to a loss of about two pounds for those on the unprocessed diet.
Metabolic testing showed that people expended more energy on the ultra-processed diet. However, that wasn’t enough to offset the increased consumption of calories. As a result, participants gained pounds and body fat. The study does have some limitations, such as slight differences in the protein content of the two diets. and the researchers plan to address such issues in their future work.
During this relatively brief study, the researchers did not observe other telltale changes associated with poor metabolic health, such as a rise in blood glucose levels or fat in the liver. While a couple of pounds might not sound like much, the extra calories and weight associated with an ultra-processed diet would, over time, add up.
So, it appears that a good place to start in reaching or maintaining a healthy weight is to follow the advice shared by all those otherwise conflicting diet plans: work to eliminate or at least reduce ultra-processed foods in your diet in favor of a balanced variety of unprocessed, nutrient-packed foods.
Reference:
[1] Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Hall KD et al. Cell Metab. 2019 May 16.
Links:
Obesity (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Healthy Eating Plan (National Heart, Lung, and Blood Institute/NIH)
Body Weight Planner (NIDDK/NIH)
Kevin D. Hall (NIDDK/NIH)
Metabolic Clinical Research Unit (NIDDK/NIH)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
‘Exercise Hormone’ Tied to Bone-Strengthening Benefits
Posted on by Dr. Francis Collins

There’s no doubt that exercise is good for us—strengthening our muscles, helping us maintain a healthy weight, maybe even boosting our moods and memories. There’s also been intriguing evidence that exercise may help build strong bones.
Now, an NIH-funded study is shedding light on the mechanism behind exercise’s bone-strengthening benefits [1]. The new work—which may lead to new approaches for treating osteoporosis, a disease that increases the risk of bone fracture—centers on a hormone called irisin that is secreted by muscles during exercise.
In a series of mouse experiments, the researchers found that irisin works directly on a common type of bone cell, stimulating the cells to produce a protein that encourages bones to thin. However, this chain of molecular events ultimately takes a turn for the better and reverses bone loss.
Bruce Spiegelman’s lab at the Dana-Farber Cancer Institute and Harvard University Medical School, Boston, first discovered the irisin hormone in 2012 [2]. In the years since, evidence has accumulated suggesting a connection between irisin and many of the benefits that come with regular workouts. For example, delivering low doses of irisin—sometimes called “the exercise hormone”—increase bone density and strength in mice.
But how does irisin act on bones? The answer hasn’t been at all clear. A major reason is the protein receptor on our cells that binds and responds to irisin wasn’t known.
In the new study reported in the journal Cell, Spiegelman’s team has now identified irisin’s protein receptor, called αVβ5 integrin. Those receptors are present on the surface of osteocytes, the most common cell type found in mature bone tissue.
The researchers went on to show that irisin helps osteocytes to live longer. It also leads the bone cells to begin secreting a protein called sclerostin, known for its role in preparing bones for remodeling and rebuilding by first breaking them down. Interestingly, previous studies also showed sclerostin levels increase in response to the mechanical stresses that come with exercise.
To further explore the role of irisin in mouse studies, the researchers gave the animals the hormone for six days. And indeed, after the treatment, the animals showed higher levels of sclerostin in their blood.
The findings suggest that irisin could form the basis of a new treatment for osteoporosis, a condition responsible for almost nine million fractures around the world each year. While it might seem strange that a treatment intended to strengthen bone would first encourage them to break down, this may be similar to the steps you have to follow when fixing up a house that has weakened timbers. And Spiegelman notes that there’s precedent for such a phenomenon in bone remodeling—treatment for osteoporosis, parathyroid hormone, also works by thinning bones before they are rebuilt.
That said, it’s not yet clear how best to target irisin for strengthening bone. In fact, locking in on the target could be a little complicated. The Speigelman lab found, for example, that mice prone to osteoporosis following the removal of their ovaries were paradoxically protected from weakening bones by the inability to produce irisin.
This new study fits right in with other promising NIH-funded efforts to explore the benefits of exercise. One that I’m particularly excited about is the Molecular Transducers of Physical Activity Consortium (MoTrPAC), which aims to develop a comprehensive map of the molecular changes that arise with physical activity, leading to a range of benefits for body and mind.
Indeed, the therapeutic potential for irisin doesn’t end with bone. In healthy people, irisin circulates throughout the body. In addition to being produced in muscle, its protein precursor is produced in the heart and brain.
The hormone also has been shown to transform energy-storing white fat into calorie-burning brown fat. In the new study, Spiegelman’s team confirms that this effect on fat also depends on the very same integrin receptors present in bone. So, these new findings will no doubt accelerate additional study in Speigelman’s lab and others to explore the many other benefits of irisin—and of exercise—including its potential to improve our moods, memory, and metabolism.
References:
[1] Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Bouxsein ML, Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM. Cell. 2018 Dec 13;175(7):1756-1768.
[2] A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. Nature. 2012 Jan 11;481(7382):463-8.
Links:
Osteoporosis (NIH)
Guide to Physical Activity (National Heart, Lung, and Blood Institute/NIH)
Spiegelman Lab (Dana-Farber Cancer Institute, Boston)
Molecular Transducers of Physical Activity in Humans (Common Fund/NIH)
Video: MoTrPAC (Common Fund)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute on Aging; National Institute of Neurological Disorders and Stroke
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: aging, Bone, bone remodeling, exercise, exercise hormone, fat, irisin, memory, metabolism, Molecular Tranducers of Physical Activity Consortium, mood, MoTrPAC, muscle, osteocyte, osteoporosis, sclerostin, thinning bones
Unraveling the Biocircuitry of Obesity
Posted on by Dr. Francis Collins

Caption: Mouse neurons (purple), with their nuclei (blue) and primary cilia (green).
Credit: Yi Wang, Vaisse Lab, UCSF
Obesity involves the complex interplay of diet, lifestyle, genetics, and even the bacteria living in the gut. But there are other less-appreciated factors that are likely involved, and a new NIH-supported study suggests one that you probably never would have imagined: antenna-like sensory projections on brain cells.
The study in mice, published in the journal Nature Genetics [1], suggests these neuronal projections, called primary cilia, are a key part of a known “hunger circuit,” which receives signals from other parts of the body to control appetite. The researchers add important evidence in mouse studies showing that changes in the primary cilia can produce a short circuit, impairing the brain’s ability to regulate appetite and leading to overeating and obesity.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Tags: ADCY3, Alström syndrome, appetite, Bardet-Biedl syndrome, brain, cell biology, childhood obesity, ciliopathies, eating, fat, food, Greenland, hunger circuit, hypothalmus, leptin, MC4R neurons, melanocortin 1 receptor gene, neurons, obesity, obesity genes, overweight, Pakistan, polydactyly, primary cilia, weight
Protein Links Gut Microbes, Biological Clocks, and Weight Gain
Posted on by Dr. Francis Collins

Caption: Lipids (red) inside mouse intestinal cells with and without NFIL3.
Credit: Lora V. Hooper, University of Texas Southwestern Medical Center, Dallas
The American epidemic of obesity is a major public health concern, and keeping off the extra pounds is a concern for many of us. Yet it can also be a real challenge for people who may eat normally but get their days and nights mixed up, including night-shift workers and those who regularly travel overseas. Why is that?
The most obvious reason is the odd hours throw a person’s 24-hour biological clock—and metabolism—out of sync. But an NIH-funded team of researchers has new evidence in mice to suggest the answer could go deeper to include the trillions of microbes that live in our guts—and, more specifically, the way they “talk” to intestinal cells. Their studies suggest that what gut microbes “say” influences the activity of a key clock-driven protein called NFIL3, which can set intestinal cells up to absorb and store more fat from the diet while operating at hours that might run counter to our fixed biological clocks.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Muscle Enzyme Explains Weight Gain in Middle Age
Posted on by Dr. Francis Collins

Thinkstock/tetmc
The struggle to maintain a healthy weight is a lifelong challenge for many of us. In fact, the average American packs on an extra 30 pounds from early adulthood to age 50. What’s responsible for this tendency toward middle-age spread? For most of us, too many calories and too little exercise definitely play a role. But now comes word that another reason may lie in a strong—and previously unknown—biochemical mechanism related to the normal aging process.
An NIH-led team recently discovered that the normal process of aging causes levels of an enzyme called DNA-PK to rise in animals as they approach middle age. While the enzyme is known for its role in DNA repair, their studies show it also slows down metabolism, making it more difficult to burn fat. To see if reducing DNA-PK levels might rev up the metabolism, the researchers turned to middle-aged mice. They found that a drug-like compound that blocked DNA-PK activity cut weight gain in the mice by a whopping 40 percent!
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Tags: aging, aging process, biochemistry, diabetes, diet, DNA repair, DNA-PK, DNA-PK inhibitor, fat, healthy weight, lymphocytes, metabolism, middle age, mitochondria, muscle, obesity, overweight, physical fitness, SCID, severe combined immunodeficiency, skeletal muscle, weight gain, weight loss, weight loss medication
Regenerative Medicine: The Promise and Peril
Posted on by Dr. Francis Collins

Caption: Scanning electron micrograph of iPSC-derived retinal pigment epithelial cells growing on a nanofiber scaffold (blue).
Credit: Sheldon Miller, Arvydas Maminishkis, Robert Fariss, and Kapil Bharti, National Eye Institute/NIH
Stem cells derived from a person’s own body have the potential to replace tissue damaged by a wide array of diseases. Now, two reports published in the New England Journal of Medicine highlight the promise—and the peril—of this rapidly advancing area of regenerative medicine. Both groups took aim at the same disorder: age-related macular degeneration (AMD), a common, progressive form of vision loss. Unfortunately for several patients, the results couldn’t have been more different.
In the first case, researchers in Japan took cells from the skin of a female volunteer with AMD and used them to create induced pluripotent stem cells (iPSCs) in the lab. Those iPSCs were coaxed into differentiating into cells that closely resemble those found near the macula, a tiny area in the center of the eye’s retina that is damaged in AMD. The lab-grown tissue, made of retinal pigment epithelial cells, was then transplanted into one of the woman’s eyes. While there was hope that there might be actual visual improvement, the main goal of this first in human clinical research project was to assess safety. The patient’s vision remained stable in the treated eye, no adverse events occurred, and the transplanted cells remained viable for more than a year.
Exciting stuff, but, as the second report shows, it is imperative that all human tests of regenerative approaches be designed and carried out with the utmost care and scientific rigor. In that instance, three elderly women with AMD each paid $5,000 to a Florida clinic to be injected in both eyes with a slurry of cells, including stem cells isolated from their own abdominal fat. The sad result? All of the women suffered severe and irreversible vision loss that left them legally or, in one case, completely blind.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Ethics, Health, Science
Tags: age-related macular degeneration, AMD, blindness, central vision, clinical research, dry AMD, eye disease, fat, fat cells, fovea, induced Pluripotent Stem cells, iPSCs, macula, macular degeneration, Nobel Prize, regenerative medicine, replacement tissue, retina, retinal pigment epithelial cells, RPE, stem cells, transplantation, vision, vision loss, wet AMD
Next Page
