fruit fly
How Neurons Make Connections
Posted on by Lawrence Tabak, D.D.S., Ph.D.

For many people, they are tiny pests. These fruit flies that sometimes hover over a bowl of peaches or a bunch of bananas. But for a dedicated community of researchers, fruit flies are an excellent model organism and source of information into how neurons self-organize during the insect’s early development and form a complex, fully functioning nervous system.
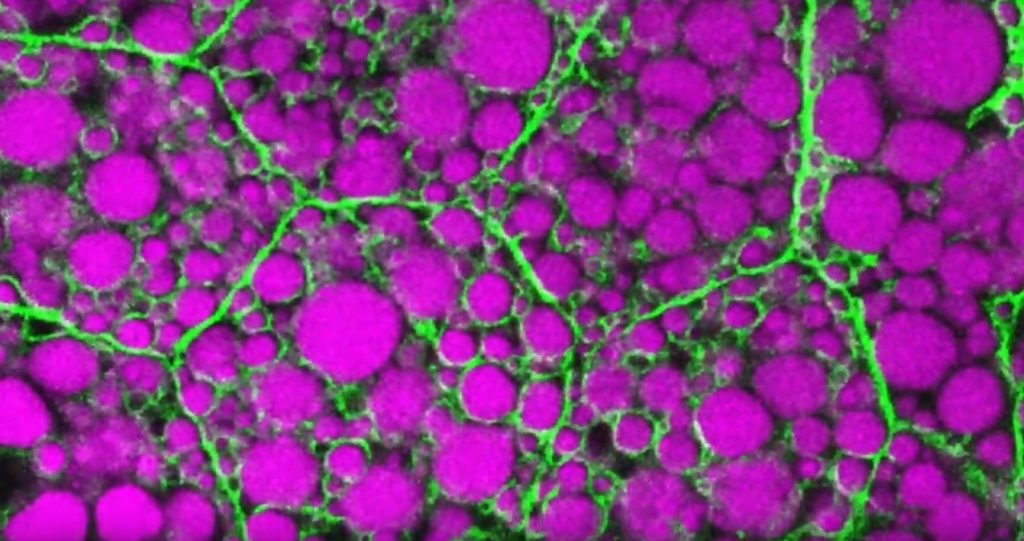
That’s the scientific story on display in this beautiful image of a larval fruit fly’s developing nervous system. Its subtext is: fundamental discoveries in the fruit fly, known in textbooks as Drosophila melanogaster, provide basic clues into the development and repair of the human nervous system. That’s because humans and fruit flies, though very distantly related through the millennia, still share many genes involved in their growth and development. In fact, 60 percent of the Drosophila genome is identical to ours.
Once hatched, as shown in this image, a larval fly uses neurons (magenta) to sense its environment. These include neurons that sense the way its body presses against the surrounding terrain, as needed to coordinate the movements of its segmented body parts and crawl in all directions.
This same set of neurons will generate painful sensations, such as the attack of a parasitic wasp. Paintbrush-like neurons in the fly’s developing head (magenta, left side) allow the insect to taste the sweetness of a peach or banana.
There is a second subtype of neurons, known as proprioceptors (green). These neurons will give the young fly its “sixth sense” understanding about where its body is positioned in space. The complete collection of developing neurons shown here are responsible for all the fly’s primary sensations. They also send these messages on to the insect’s central nervous system, which contains thousands of other neurons that are hidden from view.
Emily Heckman, now a postdoctoral researcher at the Michigan Neuroscience Institute, University of Michigan, Ann Arbor, captured this image during her graduate work in the lab of Chris Doe, University of Oregon, Eugene. For her keen eye, she received a trainee/early-career BioArt Award from the Federation of American Societies for Experimental Biology (FASEB), which each year celebrates the art of science.
The image is one of many from a much larger effort in the Doe lab that explores the way neurons that will partner find each other and link up to drive development. Heckman and Doe also wanted to know how neurons in the developing brain interconnect into integrated neural networks, or circuits, and respond when something goes wrong. To find out, they disrupted sensory neurons or forced them to take alternate paths and watched to see what would happen.
As published in the journal eLife [1], the system has an innate plasticity. Their findings show that developing sensory neurons instruct one another on how to meet up just right. If one suddenly takes an alternate route, its partner can still reach out and make the connection. Once an electrically active neural connection, or synapse, is made, the neural signals themselves slow or stop further growth. This kind of adaptation and crosstalk between neurons takes place only during a particular critical window during development.
Heckman says part of what she enjoys about the image is how it highlights that many sensory neurons develop simultaneously and in a coordinated process. What’s also great about visualizing these events in the fly embryo is that she and other researchers can track many individual neurons from the time they’re budding stem cells to when they become a fully functional and interconnected neural circuit.
So, the next time you see fruit flies hovering in the kitchen, just remember there’s more to their swarm than you think. Our lessons learned studying them will help point researchers toward new ways in people to restore or rebuild neural connections after devastating disruptions from injury or disease.
Reference:
Presynaptic contact and activity opposingly regulate postsynaptic dendrite outgrowth. Heckman EL, Doe CQ. Elife. 2022 Nov 30;11:e82093.
Links:
Research Organisms (National Institute of General Medical Sciences/NIH)
Doe Lab (University of Oregon, Eugene)
Emily Heckman (University of Michigan, Ann Arbor)
BioArt Awards (Federation of American Societies for Experimental Biology, Rockville, MD)
NIH Support: Eunice Kennedy Shriver National Institute of Child Health and Human Development
Why Flies and Humans Freeze When Startled
Posted on by Dr. Francis Collins
When faced with something unexpected and potentially ominous, like a sudden, loud noise or a threat of danger, humans often freeze before we act. This is colloquially referred to as the “deer in the headlights” phenomenon. The movie of fruit flies that you see above may help explain the ancient origins of the “startle response” and other biomechanical aspects of motion.
In this video, which shows a footrace between two flies (Drosophila melanogaster), there are no winners or losers. Their dash across the screen provides a world-class view of the biomechanics of walking in these tiny, 3 millimeter-long insects that just won’t sit still.
The fly at the top zips along at about 25 millimeters per second, the normal walking speed for Drosophila. As a six-legged hexapod, the fly walks with a “tripod gait,” alternating between its stance phase—right fore (RF), left middle (LM), and right hind (RH) —and its swing phase sequence of left fore (LF), right middle (RM), and left hind (LH).
The slowpoke at the bottom of the video clocks in at a mere 15 millimeters per second. This fly’s more-tentative gait isn’t due to an injury or a natural lack of speed. What is causing the delay is the rapid release of the chemical messenger serotonin into its nervous system, which models a startle response.
You may have already heard about serotonin because of its role in regulating mood and appetite in humans. Now, a team led by Richard S. Mann and Clare Howard, Columbia University’s Zuckerman Institute, New York, has discovered that fruit flies naturally release serotonin to turn on neural circuits that downshift and steady the speed of their gait.
As detailed recently in Current Biology [1], serotonin is active under myriad conditions to tell flies to slow things down. For example, serotonin helps flies weather the stress of extreme temperatures, conserve energy during bouts of hunger, and even walk upside down on the ceiling.
But the research team, which was supported by the NIH-led Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, found that serotonin’s most-powerful effect came during an actual startle response, prompted by a sudden, jolting vibration. Scientists suspect the release of serotonin activates motor neurons much like an emergency brake, stiffening and locking up the fly’s leg joints. When the researchers blocked the fly’s release of serotonin, it interrupted their normal startle response.
In years past, such a detailed, high-resolution “action video” of Drosophila, one of the most-popular model organisms in biology, would have been impossible to produce. Fruit flies are tiny and possess extremely high energy.
But a few years ago, the Mann lab developed the approach used in this video to bring the hurried gait of fruit flies into tight focus [2]. Their system combines an optical touch sensor and high-speed video imaging that records the footfalls of all six of a fly’s feet.
Then, using the lab’s unique software program called FlyWalker , the researchers can extract various biomechanical parameters of walking in time and space. These include step length, footprint alignment, and, as the letters in the video show, the natural sequence of a tripod gait.
Drosophila may be a very distant relative of humans. But these ubiquitous insects that sometimes buzz around our fruit bowls contain many fundamental clues into human biology, whether the area of research is genetics, nutrition, biomechanics, or even the underlying biology of the startle response.
Reference:
[1] Serotonergic Modulation of Walking in Drosophila. Howard CE, Chen CL, Tabachnik T, Hormigo R, Ramdya P, Mann RS. Curr Biol. 2019 Nov 22.
[2] Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. Mendes CS, Bartos I, Akay T, Márka S, Mann RS. Elife. 2013 Jan 8;2:e00231.
Links:
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Mann Lab (Columbia University’s Zuckerman Institute, New York)
MouseWalker Colored Feet (YouTube)
NIH Support: National Institute for Neurological Disorders and Stroke; National Institute of General Medical Sciences
Finding Beauty in the Nervous System of a Fruit Fly Larva
Posted on by Dr. Francis Collins
Wow! Click on the video. If you’ve ever wondered where those pesky flies in your fruit bowl come from, you’re looking at it right now. It’s a fruit fly larva. And this 3D movie offers never-before-seen details into proprioception—the brain’s sixth sense of knowing the body’s location relative to nearby objects or, in this case, fruit.
This live-action video highlights the movement of the young fly’s proprioceptive nerve cells. They send signals to the fly brain that are essential for tracking the body’s position in space and coordinating movement. The colors indicate the depth of the nerve cells inside the body, showing those at the surface (orange) and those further within (blue).
Such movies make it possible, for the first time, to record precisely how every one of these sensory cells is arranged within the body. They also provide a unique window into how body positions are dynamically encoded in these cells, as a segmented larva inches along in search of food.
The video was created using a form of confocal microscopy called Swept Confocally Aligned Planar Excitation, or SCAPE. It captures 3D images by sweeping a sheet of laser light back and forth across a living sample. Even better, it does this while the microscope remains completely stationary—no need for a researcher to move any lenses up or down, or hold a live sample still.
Most impressively, with this new high-speed technology, developed with support from the NIH’s BRAIN Initiative, researchers are now able to capture videos like the one seen above in record time, with each whole volume recorded in under 1/10th of a second! That’s hundreds of times faster than with a conventional microscope, which scans objects point by point.
As reported in Current Biology, the team, led by Elizabeth Hillman and Wesley Grueber, Columbia University, New York, didn’t stop at characterizing the structural details and physical movements of nerve cells involved in proprioception in a crawling larva. In another set of imaging experiments, they went a step further, capturing faint flashes of green in individual labeled nerve cells each time they fired. (You have to look very closely to see them.) With each wave of motion, proprioceptive nerve cells light up in sequence, demonstrating precisely when they are sending signals to the animal’s brain.
From such videos, the researchers have generated a huge amount of data on the position and activity of each proprioceptive nerve cell. The data show that the specific position of each cell makes it uniquely sensitive to changes in position of particular segments of a larva’s body. While most of the proprioceptive nerve cells fired when their respective body segment contracted, others were attuned to fire when a larval segment stretched.
Taken together, the data show that proprioceptive nerve cells provide the brain with a detailed sequence of signals, reflecting each part of a young fly’s undulating body. It’s clear that every proprioceptive neuron has a unique role to play in the process. The researchers now will create similar movies capturing neurons in the fly’s central nervous system.
A holy grail of the BRAIN Initiative is to capture the brain in action. With these advances in imaging larval flies, researchers are getting ever closer to understanding the coordinated activities of an organism’s complete nervous system—though this one is a lot simpler than ours! And perhaps this movie—and the anticipation of the sequels to come—may even inspire a newfound appreciation for those pesky flies that sometimes hover nearby.
Reference:
[1] Characterization of Proprioceptive System Dynamics in Behaving Drosophila Larvae Using High-Speed Volumetric Microscopy. Vaadia RD, Li W, Voleti V, Singhania A, Hillman EMC, Grueber WB. Curr Biol. 2019 Mar 18;29(6):935-944.e4.
Links:
Using Research Organisms to Study Health and Disease (National Institute of General Medical Sciences/NIH)
The Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Hillman Lab (Columbia University, New York)
Grueber Lab (Columbia University, New York)
NIH Support: National Institute of Neurological Disorders and Stroke; Eunice Kennedy Shriver National Institute of Child Health and Human Development
Students Contribute to Research Through Ovarian Art
Posted on by Dr. Francis Collins

Seeing the development of an organ under a microscope for the first time can be a truly unforgettable experience. But for a class taught by Crystal Rogers at California State University, Northridge, it can also be an award-winning moment.
This image, prepared during a biology lab course, was one of the winners in the 2018 BioArt Scientific Image & Video Competition, sponsored by the Federation of American Societies for Experimental Biology (FASEB). This colorful image shows the tip of an ovary from a fruit fly (Drosophila melanogaster), provided by Mariano Loza-Coll. You can see that the ovary is packed with oocytes (DNA stained blue). The orderly connective structure (pink) and signal-transmitting molecules like STAT (yellow) are common to egg maturation and reproductive processes in humans.
What makes this image unique among this year’s BioArt winners is that the prep work was done by undergraduate students just learning how to work in a lab. They did the tissue dissections, molecular labeling, and beautiful stainings in preparation for Rogers to “snap” the photo on her research lab’s optical-sectioning microscope.
What’s also fantastic is that many of Rogers’s students are from groups traditionally underrepresented in biomedicine. Many are considering careers in research and, from the looks of things, they are off to a beautiful start.
After teaching classes, Rogers also has an NIH-supported lab to run. She and her team study salamanders and chickens to determine how biological “glue” proteins, called cadherins, help to create neural crest cells, a critical cell type that arises very early in development [1].
For developmental biologists, it’s essential to understand what prompts these neural crest cells to migrate to locations throughout the body, from the heart to the skin to the cranium, or head. For example, cranial neural crest cells at first produce what appears to be the same generic, undifferentiated facial template in vertebrate species. And yet, neural crest cells and the surrounding ectodermal cells go on to generate craniofacial structures as distinct as the beak of a toucan, the tusk of a boar, or the horn of a rhinoceros.
But if the organ of interest is an ovary, the fruit fly has long been a go-to organism to learn more. Not only does the fruit fly open a window into ovarian development and health issues like infertility, it showcases the extraordinary beauty of biology.
Reference:
[1] A catenin-dependent balance between N-cadherin and E-cadherin controls neuroectodermal cell fate choices. Rogers CD, Sorrells LK, Bronner ME. Mech Dev. 2018 Aug;152:44-56.
Links:
Rogers Lab (California State University, Northridge)
BioArt Scientific Image & Video Competition (Federation of American Societies for Experimental Biology, Bethesda, MD)
NIH Support: Eunice Kennedy Shriver National Institute of Child Health and Human Development
A Fantastic WALS Lecture
Posted on by Dr. Francis Collins
Creative Minds: Building a CRISPR Gene Drive Against Malaria
Posted on by Dr. Francis Collins

Valentino Gantz/Credit: Erik Jepsen
Researchers have used Drosophila melanogaster, the common fruit fly that sometimes hovers around kitchens, to make seminal discoveries involving genetics, the nervous system, and behavior, just to name a few. Could a new life-saving approach to prevent malaria be next? Valentino Gantz, a researcher at the University of California, San Diego, is on a path to answer that question.
Gantz has received a 2016 NIH Director’s Early Independence Award to use Drosophila to hone a new bioengineered tool that acts as a so-called “gene drive,” which spreads a new genetically encoded trait through a population much faster than would otherwise be possible. The lessons learned while working with flies will ultimately be applied to developing a more foolproof system for use in mosquitoes with the hope of stopping the transmission of malaria and potentially other serious mosquito-borne diseases.
Creative Minds: A New Mechanism for Epigenetics?
Posted on by Dr. Francis Collins
To learn more about how DNA and inheritance works, Keith Maggert has spent much of his nearly 30 years as a researcher studying what takes place not just within the DNA genome but also the subtle modifications of it. That’s where a stable of enzymes add chemical marks to DNA, turning individual genes on or off without changing their underlying sequence. What’s really intrigued Maggert is these “epigenetic” modifications are maintained through cell division and can even get passed down from parent to child over many generations. Like many researchers, he wants to know how it happens.
Maggert thinks there’s more to the story than scientists have realized. Now an associate professor at the University of Arizona College of Medicine, Tucson, he suspects that a prominent subcellular structure in the nucleus called the nucleolus also exerts powerful epigenetic effects. What’s different about the nucleolus, Maggert proposes, is it doesn’t affect genes one by one, a focal point of current epigenetic research. He thinks under some circumstances its epigenetic effects can activate many previously silenced, or “off” genes at once, sending cells and individuals on a different path toward health or disease.
Maggert has received a 2016 NIH Director’s Transformative Research Award to pursue this potentially new paradigm. If correct, it would transform current thinking in the field and provide an exciting new perspective to track epigenetics and its contributions to a wide range of human diseases, including cancer, cardiovascular disease, and neurodegenerative disorders.
Snapshots of Life: Hardwired to Sense Food Texture
Posted on by Dr. Francis Collins
It’s a problem that parents know all too well: a child won’t eat because their oatmeal is too slimy or a slice of apple is too hard. Is the kid just being finicky? Or is there a biological basis for disliking food based on its texture? This image, showing the tongue (red) of a fruit fly (Drosophila melanogaster), provides some of the first evidence that biology could indeed play a role [1].
The image shows a newly discovered mechanosensory nerve cell (green), which is called md-L, short for multidendritic neuron in the labellum. When the fly extends its tongue to eat, the hair bristles (short red lines) on its surface bend in proportion to the consistency of the food. If a bristle is bent hard enough, the force is detected at its base by one of the arms of an md-L neuron. In response, the arm shoots off an electrical signal that’s relayed to the central part of the neuron and onward to the brain via the outgoing informational arm, or axon.