metabolic syndrome
Why When You Eat Might Be as Important as What You Eat
Posted on by Dr. Francis Collins

About 1 in 3 American adults have metabolic syndrome, a group of early warning signs for increased risk of type 2 diabetes, heart disease, and stroke. To help avoid such health problems, these folks are often advised to pay close attention to the amount and type of foods they eat. And now it seems there may be something else to watch: how food intake is spaced over a 24-hour period.
In a three-month pilot study, NIH-funded researchers found that when individuals with metabolic syndrome consumed all of their usual daily diet within 10 hours—rather than a more customary span of about 14 hours—their early warning signs improved. Not only was a longer stretch of daily fasting associated with moderate weight loss, in some cases, it was also tied to lower blood pressure, lower blood glucose levels, and other improvements in metabolic syndrome.
The study, published in Cell Metabolism, is the result of a joint effort by Satchidananda Panda, Salk Institute for Biological Sciences, La Jolla, CA, and Pam R. Taub, University of California, San Diego [1]. It was inspired by Panda’s earlier mouse studies involving an emerging dietary intervention, called time-restricted eating (TRE), which attempts to establish a consistent daily cycle of feeding and fasting to create more stable rhythms for the body’s own biological clock [2, 3].
But would observations in mice hold true for humans? To find out, Panda joined forces with Taub, a cardiologist and physician-scientist. The researchers enlisted 19 men and women with metabolic syndrome, defined as having three or more of five specific risk factors: high fasting blood glucose, high blood pressure, high triglyceride levels, low “good” cholesterol, and/or extra abdominal fat. Most participants were obese and taking at least one medication to help manage their metabolic risk factors.
In the study, participants followed one rule: eat anything that you want, just do so over a 10-hour period of your own choosing. So, for the next three months, these folks logged their eating times and tracked their sleep using a special phone app created by the research team. They also wore activity and glucose monitors.
By the pilot study’s end, participants following the 10-hour limitation had lost on average 3 percent of their weight and about 3 percent of their abdominal fat. They also lowered their cholesterol and blood pressure. Although this study did not find 10-hour TRE significantly reduced blood glucose levels in all participants, those with elevated fasting blood glucose did have improvement. In addition, participants reported other lifestyle improvements, including better sleep.
The participants generally saw their metabolic health improve without skipping meals. Most chose to delay breakfast, waiting about two hours after they got up in the morning. They also ate dinner earlier, about three hours before going to bed—and then did no late night snacking.
After the study, more than two-thirds reported that they stuck with the 10-hour eating plan at least part-time for up to a year. Some participants were able to cut back or stop taking cholesterol and/or blood-pressure-lowering medications.
Following up on the findings of this small study, Taub will launch a larger NIH-supported clinical trial involving 100 people with metabolic syndrome. Panda is now exploring in greater detail the underlying biology of the metabolic benefits observed in the mice following TRE.
For people looking to improve their metabolic health, it’s a good idea to consult with a doctor before making significant changes to one’s eating habits. But the initial data from this study indicate that, in addition to exercising and limiting portion size, it might also pay to watch the clock.
References:
[1] Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleisher JG, Panda S, Taub PR. Cell Metab. 2019 Jan 7; 31: 1-13. Epub 2019 Dec 5.
[2] Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Cell Metab. 2012 Jun 6;15(6):848-60.
[3] Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Chaix A, Zarrinpar A, Miu P, Panda S. Cell Metab. 2014 Dec 2;20(6):991-1005.
Links:
Metabolic Syndrome (National Heart, Lung, and Blood Institute/NIH)
Obesity (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Body Weight Planner (NIDDK/NIH)
Satchidananda Panda (Salk Institute for Biological Sciences, La Jolla, CA)
Taub Research Group (University of California, San Diego)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: bad cholesterol, biological clock, blood glucose, blood pressure, circadian rhythms, diet, fasting, fat, food, lipids, metabolic syndrome, metabolism, obesity, pilot study, sleep, time-restricted eating, TRE, triglycerides, weight loss
Cardiometabolic Disease: Big Data Tackles a Big Health Problem
Posted on by Dr. Francis Collins
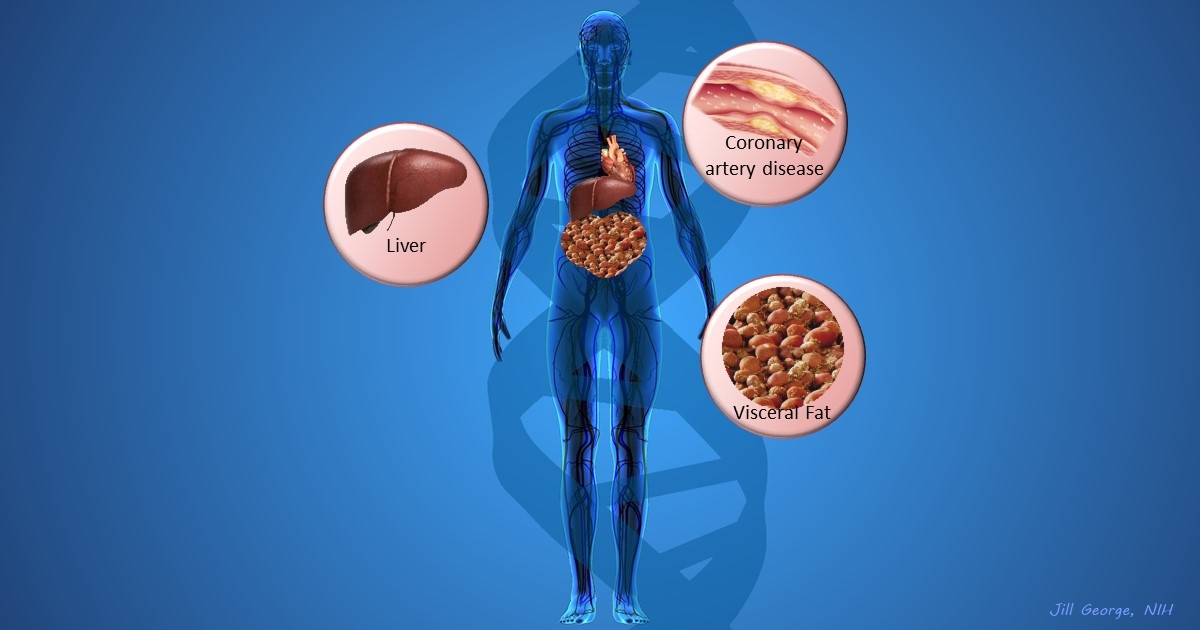
More and more studies are popping up that demonstrate the power of Big Data analyses to get at the underlying molecular pathology of some of our most common diseases. A great example, which may have flown a bit under the radar during the summer holidays, involves cardiometabolic disease. It’s an umbrella term for common vascular and metabolic conditions, including hypertension, impaired glucose and lipid metabolism, excess belly fat, and inflammation. All of these components of cardiometabolic disease can increase a person’s risk for a heart attack or stroke.
In the study, an international research team tapped into the power of genomic data to develop clearer pictures of the complex biocircuitry in seven types of vascular and metabolic tissue known to be affected by cardiometabolic disease: the liver, the heart’s aortic root, visceral abdominal fat, subcutaneous fat, internal mammary artery, skeletal muscle, and blood. The researchers found that while some circuits might regulate the level of gene expression in just one tissue, that’s often not the case. In fact, the researchers’ computational models show that such genetic circuitry can be organized into super networks that work together to influence how multiple tissues carry out fundamental life processes, such as metabolizing glucose or regulating lipid levels. When these networks are perturbed, perhaps by things like inherited variants that affect gene expression, or environmental influences such as a high-carb diet, sedentary lifestyle, the aging process, or infectious disease, the researchers’ modeling work suggests that multiple tissues can be affected, resulting in chronic, systemic disorders including cardiometabolic disease.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Tags: bad cholesterol, big data, bioinformatics, blood lipids, cardiometabolic disease, cardiovascular disease, coronary artery disease, coronary bypass surgery, drug delivery, eQTL, Estonia, fat, gemomics, gene networks, gene variants, GWAS, hyperlipidemia, hypertension, LDL, liver, metabolic syndrome, NHGRI GWAS Catalog, PCSK9, PMI, Precision Medicine Initiative Cohort Program, STARNET, Sweden, systems biology, systems genetics, type 2 diabetes, visceral abdominal fat, visceral fat
