chemotherapy
Wearable Sensor Promises More Efficient Early Cancer Drug Development
Posted on by Lawrence Tabak, D.D.S., Ph.D.

Wearable electronic sensors hold tremendous promise for improving human health and wellness. That promise already runs the gamut from real-time monitoring of blood pressure and abnormal heart rhythms to measuring alcohol consumption and even administering vaccines.
Now a new study published in the journal Science Advances [1] demonstrates the promise of wearables also extends to the laboratory. A team of engineers has developed a flexible, adhesive strip that, at first glance, looks like a Band-Aid. But this “bandage” actually contains an ultra-sensitive, battery-operated sensor that’s activated when placed on the skin of mouse models used to study possible new cancer drugs.
This sensor is so sensitive that it can detect, in real time, changes in the size of a tumor down to one-hundredth of a millimeter. That’s about the thickness of the plastic cling wrap you likely have in your kitchen! The device beams those measures to a smartphone app, capturing changes in tumor growth minute by minute over time.
The goal is to determine much sooner—and with greater automation and precision—which potential drug candidates undergoing early testing in the lab best inhibit tumor growth and, consequently, should be studied further. In their studies in mouse models of cancer, researchers found the new sensor could detect differences between tumors treated with an active drug and those treated with a placebo within five hours. Those quick results also were validated using more traditional methods to confirm their accuracy.
The device is the work of a team led by Alex Abramson, a former post-doc with Zhenan Bao, Stanford University’s School of Engineering, Palo Alto, CA. Abramson has since launched his own lab at the Georgia Institute of Technology, Atlanta.
The Stanford team began looking for a technological solution after realizing the early testing of potential cancer drugs typically requires researchers to make tricky measurements using pincer-like calipers by hand. Not only is the process tedious and slow, it’s less than an ideal way to capture changes in soft tissues with the desired precision. The imprecision can also lead to false leads that won’t pan out further along in the drug development pipeline, at great time and expense to their developers.
To refine the process, the NIH-supported team turned to wearable technology and recent advances in flexible electronic materials. They developed a device dubbed FAST (short for Flexible Autonomous Sensor measuring Tumors). Its sensor, embedded in a skin patch, is composed of a flexible and stretchable, skin-like polymer with embedded gold circuitry.
Here’s how FAST works: Coated on top of the polymer skin patch is a layer of gold. When stretched, it forms small cracks that change the material’s electrical conductivity. As the material stretches, even slightly, the number of cracks increases, causing the electronic resistance in the sensor to increase as well. As the material contracts, any cracks come back together, and conductivity improves.
By picking up on those changes in conductivity, the device measures precisely the strain on the polymer membrane—an indication of whether the tumor underneath is stable, growing, or shrinking—and transmits that data to a smartphone. Based on that information, potential therapies that are linked to rapid tumor shrinkage can be fast-tracked for further study while those that allow a tumor to continue growing can be cast aside.
The researchers are continuing to test their sensor in more cancer models and with more therapies to extend these initial findings. Already, they have identified at least three significant advantages of their device in early cancer drug testing:
• FAST is non-invasive and captures precise measurements on its own.
• It can provide continuous monitoring, for weeks, months, or over the course of study.
• The flexible sensor fully surrounds the tumor and can therefore detect 3D changes in shape that would be hard to pick up otherwise in real-time with existing technologies.
By now, you are probably asking yourself: Could FAST also be applied as a wearable for cancer patients to monitor in real-time whether an approved chemotherapy regimen is working? It is too early to say. So far, FAST has not been tested in people. But, as highlighted in this paper, FAST is off to, well, a fast start and points to the vast potential of wearables in human health, wellness, and also in the lab.
Reference:
[1] A flexible electronic strain sensor for the real-time monitoring of tumor regression. Abramson A, Chan CT, Khan Y, Mermin-Bunnell A, Matsuhisa N, Fong R, Shad R, Hiesinger W, Mallick P, Gambhir SS, Bao Z. Sci Adv. 2022 Sep 16;8(37):eabn6550.
Links:
Stanford Wearable Electronics Initiative (Stanford University, Palo Alto, CA)
Bao Group (Stanford University)
Abramson Lab (Georgia Institute of Technology, Atlanta)
NIH Support: National Institute of Biomedical Imaging and Bioengineering
Seven Questions for a Rare Disease Warrior
Posted on by Dr. Francis Collins

Credit: National Disease Research Interchange, Philadelphia
Tomorrow is Rare Disease Day at NIH, marking the 12th year that this annual event has been held on the NIH campus. Similar gatherings have been organized independently around the world this week, all to raise awareness for the nearly 7,000 rare diseases, some affecting just a few dozen people. But, collectively, rare diseases are hardly rare. One in 10 Americans has a rare disease (defined as affecting 200,000 or fewer individuals in the US), and about half are children. Without needed treatments, about 30 percent of these children will die by age 5.
To join everyone in raising awareness, I wanted to feature on my blog a unique perspective about rare diseases, and David Fajgenbaum certainly has one. Fajgenbaum is an immunologist and NIH grantee at the Perelman School of Medicine, University of Pennsylvania, Philadelphia. When Fajgenbaum isn’t running studies or clinical trials, he must remain vigilant of his own health. Fajgenbaum has a rare disease called idiopathic multicentric Castleman disease (iMCD), and this devastating condition, which emerged while he was in medical school, nearly claimed his life several times.
Now 34 years old and in a long remission, Fajgenbaum can discuss rare diseases as a doctor, as a patient, as a researcher, and as an advocate. His personal journey, published in his recent book Chasing My Cure, is a gripping read. Fajgenbaum was kind enough to answer a few of my questions on rare diseases and share some of his lessons learned.
The last time that I saw you, David, you looked great. How long have you been in remission?
I have been in remission for 73.83 months. I say 73.83, because I know that I can’t round up—I may relapse tomorrow. But I also refuse to round down because so many colleagues and I have worked so hard for every day of remission for me and other patients with my disease.
For me, every day is particularly special, because I never thought that I would be alive this long. As you know, I became deathly ill during medical school in 2010 and even had my last rites read to me when my doctors didn’t think I would survive. I was eventually diagnosed with idiopathic multicentric Castleman disease (iMCD), which is like a deadly cross between cancer and autoimmunity. Chemotherapy saved my life, but I would go on to have four near-death relapses.
After one of those relapses, I got out of the hospital and dedicated my life to conducting iMCD research and co-founded the Castleman Disease Collaborative Network (CDCN). Later, I identified a particular cellular pathway called mTOR that was highly active in my samples. I began testing on myself an mTOR inhibitor [sirolimus]—developed 30 years before and approved for kidney transplantation but never considered for iMCD. It’s this drug that has kept me in remission for the last 73.83 months and helped other people. During this time, I’ve been able to marry my wife, have a daughter, help launch a new center at Penn specializing in rare diseases, and write a book to share my personal journey with others.
As a physician-scientist and as a person with a rare disease, what have you learned about the biomedical research process?
I’ve learned so much, but I’d like to highlight three lessons in particular. First, we must leverage all perspectives to prioritize research and give us the best chance of translating research into meaningful breakthroughs. The traditional approach to rare disease research involves a subset of researchers within a rare disease field submitting their best ideas for funding and a panel selecting the best applicant.
Through the CDCN, we’ve spearheaded a new approach called the Collaborative Network Approach, where we crowdsource research questions from the entire community of patients, physicians, and researchers (not just a subset of researchers) and then recruit the best researchers in the world (not just from within the Castleman disease field) to perform the prioritized studies. We’re now working to improve and spread this approach to other diseases.
Second, collaboration between all players is critical. Patient advocacy groups are uniquely positioned to serve as the glue between all stakeholders. Researchers and physicians need to share ideas, data, and samples with one another. Patients need to be actively involved in research question prioritization and study design. Biopharma and the Food and Drug Administration (FDA) need to be engaged early in the process of research discoveries and drug development.
Third, we must leverage all 1,500-plus, existing FDA-approved drugs to help as many patients without any options as quickly as possible. As you know, less than 5 percent of the nearly 7,000 rare diseases have an FDA-approved therapy, but many diseases share similar cellular and genetic defects that could make them susceptible to the same drugs. I’m literally alive today thanks to a drug developed for another disease. How many of the drugs approved for one disease may be effective for many of the 7,000 diseases without any? I don’t know the answer, but I hope we can begin to address this important question and incentivize repurposing.
In your experience, how can people with rare diseases help to advance progress for their conditions?
There is so much work to be done for so many rare diseases. Sometimes it can feel so overwhelming and like “what can I really do?”
But I’ve learned that there are so many ways that we can each contribute and so many incredible examples of advocates who have made a difference for themselves and those that they love. Cystic fibrosis and chordoma are just two of many examples where patient-advocates have been critical partners in transforming their diseases.
People with rare diseases can raise funds for research. Every dollar truly counts. We can work with existing organizations for our disease to ensure that those funds are distributed as efficiently and effectively as possible. If there are major gaps within our rare disease fields that aren’t being addressed by existing organizations, we can start new rare disease organizations (but we should try to avoid this whenever possible). We can contribute samples and data towards research, participate in clinical trials, and share with other patients about our experiences. We can advocate for new drug development and repurposing already-FDA approved drugs for our diseases.
What would you tell other researchers who are studying rare diseases?
I would tell other rare disease researchers that you are doing such important work. You give us hope that a treatment can be identified that will change our lives. It’s an incredible responsibility and incredibly stressful. There are unfortunately far too many scientific questions and diseases with major unmet need for any of us to compete over the use of samples and data. We have to share these within our fields. And we must also work together across rare diseases. We can’t continue to reinvent the wheel; we must share learnings with one another
I enjoyed doing the CastleMan Warrior Flex with you. Tell us more about what it represents?
Doing the CastleMan Warrior Flex with you is one of my favorite pictures. In fact, it’s hanging up in my office.
Castleman disease was named after Dr. Benjamin Castleman, who first described our disease in 1954. We have repurposed the “Castleman” name to be a “CastleMan Warrior” (below is our cartoon mascot). We do the “CastleMan Warrior” Flex to raise awareness for Castleman disease and rare diseases generally—we’re all warriors in the rare disease space.

What are your future plans as a rare disease advocate and as a researcher?
We’ve made a lot of progress for Castleman disease: we’ve advanced our findings about mTOR towards a clinical trial, gained approval for the treatment siltuximab for iMCD, developed diagnostic criteria and treatment guidelines, and invested about $1.5 million into Castleman disease research, which has led to over $7 million in additional funding from other sources.
But we still have important work ahead of us. The treatments sirolimus and siltuximab work for only a portion of all iMCD patients. We need to identify more effective treatments for all forms of Castleman disease.
I will continue to study Castleman disease and other diseases at the intersection of autoimmunity and oncology to gain insights into how the immune system works in myriad diseases. In parallel, I will continue to advocate for the adoption of the “Collaborative Network Approach” to crowdsource all stakeholder perspectives as well as for new models for drug repurposing.
Any other issues that you’d like to address?
I feel a responsibility to share with the world the lessons that I’ve learned about life from nearly dying five times. This is a major reason that I wrote my book.
One lesson that I think about a lot is related to my growing up playing football. Some of my games were extended into an overtime period to decide the outcome. In overtime, every second counts and you’re totally focused on what’s important. I’ve lived with that exact same feeling ever since I had my last rites read to me.
I’ve also learned that humor can be incredibly powerful. You may think that a good laugh may be the last thing that you’d want to do when you’re dying in the ICU. But laughing with the people that I love actually helped me feel like I could transcend my illness, and it helped to connect us.
My greatest regrets on my deathbed were not things that I had done or said. I regretted what I didn’t do or didn’t say and that I would no longer be able to do. I now follow the motto: “Think It, Do It.” In other words, we should reflect on what we’re hoping for and then turn our hopes into action.
Finally, I’ve learned that it really takes a strong team to make a difference in the world, especially against diseases. If it was just me on my own, we would have made less than 1 percent of the progress that’s been achieved. I hope that all rare disease warriors will join together into strong teams, armies even, and make a difference in the world.
Links:
Multicentric Castleman Disease (Genetic and Rare Diseases Information Center/NIH)
Castleman Disease Collaborative Network (Paso Robles, CA)
His Doctors Were Stumped. Then He Took Over (New York Times, February 4, 2017)
Video: Chasing My Cure: Dr. David Fajgenbaum’s Lessons From His Rare Disease And On Finding Cures For Others (Exponential Medicine, November 4, 2019)
Rare Disease Day at NIH 2020 (National Center for Advancing Translational Sciences/NIH)
Giving Thanks for Biomedical Research
Posted on by Dr. Francis Collins
This Thanksgiving, Americans have an abundance of reasons to be grateful—loving family and good food often come to mind. Here’s one more to add to the list: exciting progress in biomedical research. To check out some of that progress, I encourage you to watch this short video, produced by NIH’s National Institute of Biomedical Imaging and Engineering (NIBIB), that showcases a few cool gadgets and devices now under development.
Among the technological innovations is a wearable ultrasound patch for monitoring blood pressure [1]. The patch was developed by a research team led by Sheng Xu and Chonghe Wang, University of California San Diego, La Jolla. When this small patch is worn on the neck, it measures blood pressure in the central arteries and veins by emitting continuous ultrasound waves.
Other great technologies featured in the video include:
• Laser-Powered Glucose Meter. Peter So and Jeon Woong Kang, researchers at Massachusetts Institute of Technology (MIT), Cambridge, and their collaborators at MIT and University of Missouri, Columbia have developed a laser-powered device that measures glucose through the skin [2]. They report that this device potentially could provide accurate, continuous glucose monitoring for people with diabetes without the painful finger pricks.
• 15-Second Breast Scanner. Lihong Wang, a researcher at California Institute of Technology, Pasadena, and colleagues have combined laser light and sound waves to create a rapid, noninvasive, painless breast scan. It can be performed while a woman rests comfortably on a table without the radiation or compression of a standard mammogram [3].
• White Blood Cell Counter. Carlos Castro-Gonzalez, then a postdoc at Massachusetts Institute of Technology, Cambridge, and colleagues developed a portable, non-invasive home monitor to count white blood cells as they pass through capillaries inside a finger [4]. The test, which takes about 1 minute, can be carried out at home, and will help those undergoing chemotherapy to determine whether their white cell count has dropped too low for the next dose, avoiding risk for treatment-compromising infections.
• Neural-Enabled Prosthetic Hand (NEPH). Ranu Jung, a researcher at Florida International University, Miami, and colleagues have developed a prosthetic hand that restores a sense of touch, grip, and finger control for amputees [5]. NEPH is a fully implantable, wirelessly controlled system that directly stimulates nerves. More than two years ago, the FDA approved a first-in-human trial of the NEPH system.
If you want to check out more taxpayer-supported innovations, take a look at NIBIB’s two previous videos from 2013 and 2018 As always, let me offer thanks to you from the NIH family—and from all Americans who care about the future of their health—for your continued support. Happy Thanksgiving!
References:
[1] Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Wang C, Li X, Hu H, Zhang, L, Huang Z, Lin M, Zhang Z, Yun Z, Huang B, Gong H, Bhaskaran S, Gu Y, Makihata M, Guo Y, Lei Y, Chen Y, Wang C, Li Y, Zhang T, Chen Z, Pisano AP, Zhang L, Zhou Q, Xu S. Nature Biomedical Engineering. September 2018, 687-695.
[2] Evaluation of accuracy dependence of Raman spectroscopic models on the ratio of calibration and validation points for non-invasive glucose sensing. Singh SP, Mukherjee S, Galindo LH, So PTC, Dasari RR, Khan UZ, Kannan R, Upendran A, Kang JW. Anal Bioanal Chem. 2018 Oct;410(25):6469-6475.
[3] Single-breath-hold photoacoustic computed tomography of the breast. Lin L, Hu P, Shi J, Appleton CM, Maslov K, Li L, Zhang R, Wang LV. Nat Commun. 2018 Jun 15;9(1):2352.
[4] Non-invasive detection of severe neutropenia in chemotherapy patients by optical imaging of nailfold microcirculation. Bourquard A, Pablo-Trinidad A, Butterworth I, Sánchez-Ferro Á, Cerrato C, Humala K, Fabra Urdiola M, Del Rio C, Valles B, Tucker-Schwartz JM, Lee ES, Vakoc BJ9, Padera TP, Ledesma-Carbayo MJ, Chen YB, Hochberg EP, Gray ML, Castro-González C. Sci Rep. 2018 Mar 28;8(1):5301.
[5] Enhancing Sensorimotor Integration Using a Neural Enabled Prosthetic Hand System
Links:
Sheng Xu Lab (University of California San Diego, La Jolla)
So Lab (Massachusetts Institute of Technology, Cambridge)
Lihong Wang (California Institute of Technology, Pasadena)
Video: Lihong Wang: Better Cancer Screenings
Carlos Castro-Gonzalez (Madrid-MIT M + Visión Consortium, Cambridge, MA)
Video: Carlos Castro-Gonzalez (YouTube)
Ranu Jung (Florida International University, Miami)
Video: New Prosthetic System Restores Sense of Touch (Florida International)
NIH Support: National Institute of Biomedical Imaging and Bioengineering; National Institute of Neurological Diseases and Stroke; National Heart, Lung, and Blood Institute; National Cancer Institute; Common Fund
Caught on Video: Cancer Cells in Act of Cannibalism
Posted on by Dr. Francis Collins
Tumors rely on a variety of tricks to grow, spread, and resist our best attempts to destroy them. Now comes word of yet another of cancer’s surprising stunts: when chemotherapy treatment hits hard, some cancer cells survive by cannibalizing other cancer cells.
Researchers recently caught this ghoulish behavior on video. In what, during this Halloween season, might look a little bit like The Blob, you can see a down-for-the-count breast cancer cell (green), treated earlier with the chemotherapy drug doxorubicin, gobbling up a neighboring cancer cell (red). The surviving cell delivers its meal to internal compartments called lysosomes, which digest it in a last-ditch effort to get some nourishment and keep going despite what should have been a lethal dose of a cancer drug.
Crystal Tonnessen-Murray, a postdoctoral researcher in the lab of James Jackson, Tulane University School of Medicine, New Orleans, captured these dramatic interactions using time-lapse and confocal microscopy. When Tonnessen-Murray saw the action, she almost couldn’t believe her eyes. Tumor cells eating tumor cells wasn’t something that she’d learned about in school.
As the NIH-funded team described in the Journal of Cell Biology, these chemotherapy-treated breast cancer cells were not only cannibalizing their neighbors, they were doing it with remarkable frequency [1]. But why?
A possible explanation is that some cancer cells resist chemotherapy by going dormant and not dividing. The new study suggests that while in this dormant state, cannibalism is one way that tumor cells can keep going.
The study also found that these acts of cancer cell cannibalism depend on genetic programs closely resembling those of immune cells called macrophages. These scavenging cells perform their important protective roles by gobbling up invading bacteria, viruses, and other infectious microbes. Drug-resistant breast cancer cells have apparently co-opted similar programs in response to chemotherapy but, in this case, to eat their own neighbors.
Tonnessen-Murray’s team confirmed that cannibalizing cancer cells have a survival advantage. The findings suggest that treatments designed to block the cells’ cannibalistic tendencies might hold promise as a new way to treat otherwise hard-to-treat cancers. That’s a possibility the researchers are now exploring, although they report that stopping the cells from this dramatic survival act remains difficult.
Reference:
[1] Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. Tonnessen-Murray CA, Frey WD, Rao SG, Shahbandi A, Ungerleider NA, Olayiwola JO, Murray LB, Vinson BT, Chrisey DB, Lord CJ, Jackson JG. J Cell Biol. 2019 Sep 17.
Links:
Breast Cancer (National Cancer Institute/NIH)
James Jackson (Tulane University School of Medicine, New Orleans)
NIH Support: National Institute of General Medical Sciences
Body-on-a-Chip Device Predicts Cancer Drug Responses
Posted on by Dr. Francis Collins
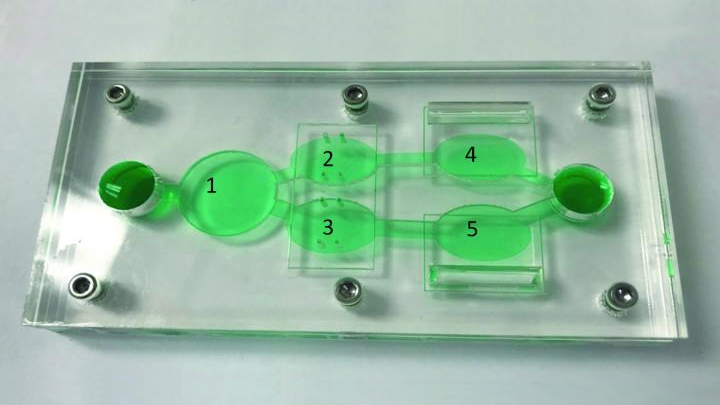
Researchers continue to produce impressive miniature human tissues that resemble the structure of a range of human organs, including the livers, kidneys, hearts, and even the brain. In fact, some researchers are now building on this success to take the next big technological step: placing key components of several miniature organs on a chip at once.
These body-on-a-chip (BOC) devices place each tissue type in its own pea-sized chamber and connect them via fluid-filled microchannels into living, integrated biological systems on a laboratory plate. In the photo above, the BOC chip is filled with green fluid to make it easier to see the various chambers. For example, this easy-to-reconfigure system can make it possible to culture liver cells (chamber 1) along with two cancer cell lines (chambers 3, 5) and cardiac function chips (chambers 2, 4).
Researchers circulate blood-mimicking fluid through the chip, along with chemotherapy drugs. This allows them to test the agents’ potential to fight human cancer cells, while simultaneously gathering evidence for potential adverse effects on tissues placed in the other chambers.
This BOC comes from a team of NIH-supported researchers, including James Hickman and Christopher McAleer, Hesperos Inc., Orlando, FL. The two were challenged by their Swiss colleagues at Roche Pharmaceuticals to create a leukemia-on-a-chip model. The challenge was to see whether it was possible to reproduce on the chip the known effects and toxicities of diclofenac and imatinib in people.
As published in Science Translational Medicine, they more than met the challenge. The researchers showed as expected that imatinib did not harm liver cells [1]. But, when treated with diclofenac, liver cells on the chip were reduced in number by about 30 percent, an observation consistent with the drug’s known liver toxicity profile.
As a second and more challenging test, the researchers reconfigured the BOC by placing a multi-drug resistant vulva cancer cell line in one chamber and, in another, a breast cancer cell line that responded to drug treatment. To explore side effects, the system also incorporated a chamber with human liver cells and two others containing beating human heart cells, along with devices to measure the cells’ electrical and mechanical activity separately.
These studies showed that tamoxifen, commonly used to treat breast cancer, indeed killed a significant number of the breast cancer cells on the BOC. But, it only did so after liver cells on the chip processed the tamoxifen to produce its more active metabolite!
Meanwhile, tamoxifen alone didn’t affect the drug-resistant vulva cancer cells on the chip, whether or not liver cells were present. This type of cancer cell has previously been shown to pump the drug out through a specific channel. Studies on the chip showed that this form of drug resistance could be overcome by adding a second drug called verapamil, which blocks the channel.
Both tamoxifen alone and the combination treatment showed some off-target effects on heart cells. While the heart cells survived the treatment, they contracted more slowly and with less force. The encouraging news was that the heart cells bounced back from the tamoxifen-only treatment within three days. But when the drug-drug combination was tested, the cardiac cells did not recover their function during the same time period.
What makes advances like this especially important is that only 1 in 10 drug candidates entering human clinical trials ultimately receives approval from the Food and Drug Administration (FDA) [2]. Often, drug candidates fail because they prove toxic to the human brain, liver, kidneys, or other organs in ways that preclinical studies in animals didn’t predict.
As BOCs are put to work in testing new drug candidates and especially treatment combinations, the hope is that we can do a better job of predicting early on which chemical compounds will prove safe and effective in humans. For those drug candidates that are ultimately doomed, “failing early” is key to reducing drug development costs. By culturing an individual patient’s cells in the chambers, BOCs also may be used to help doctors select the best treatment option for that particular patient. The ultimate goal is to accelerate the translation of basic discoveries into clinical breakthroughs. For more information about tissue chips, take a look at NIH’s Tissue Chip for Drug Screening program.
References:
[1] Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. McAleer CW, Long CJ, Elbrecht D, Sasserath T, Bridges LR, Rumsey JW, Martin C, Schnepper M, Wang Y, Schuler F, Roth AB, Funk C, Shuler ML, Hickman JJ. Sci Transl Med. 2019 Jun 19;11(497).
[2] Clinical development success rates for investigational drugs. Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Nat Biotechnol. 2014 Jan;32(1):40-51.
Links:
Tissue Chip for Drug Screening (National Center for Advancing Translational Sciences/NIH)
James Hickman (Hesperos, Inc., Orlando, FL)
NIH Support: National Center for Advancing Translational Sciences
Personalized Combination Therapies Yield Better Cancer Outcomes
Posted on by Dr. Francis Collins

Gratifying progress has been made recently in an emerging area of cancer medicine called precision oncology. It’s a bold attempt to target treatment to the very genes and molecules driving a cancer, aiming to slow or even halt its growth. But there’s always more to learn. Now comes evidence that, while a single well-matched drug might be good, a tailored combination of drugs that attack a cancer in multiple ways at once might be even better.
The findings come from the I-PREDICT clinical trial, which treated people with advanced cancer who hadn’t benefited from previous therapy [1]. The NIH-funded team found that analyzing a tumor’s unique genetic and molecular profile provided enough information to recommend individualized combination therapies to patients. What’s more, patients who followed their individualized combination therapies most closely lived longer, with longer periods of progression-free disease, than did those who took fewer of the recommended drugs.
In most previous clinical trials of precision oncology, researchers have relied on a tumor’s unique profile to identify a single, well-matched drug to treat each patient. But cancer is complex, and, just as with certain infectious diseases, tumors commonly develop resistance to a single drug.
In the trial reported in Nature Medicine, researchers led by Razelle Kurzrock and Jason Sicklick, University of California, San Diego, wondered if they could improve treatment responses by tailoring combinations of cancer drugs to target as many molecular and genetic changes in a person’s cancer as possible.
To test the potential for this strategy to work, the researchers enrolled 83 people with various cancers that had advanced despite previous treatment. Tumor tissue from each patient was run through a comprehensive battery of tests, and researchers sequenced hundreds of genes to look for telltale alterations in their DNA.
They also looked for evidence that a cancer had defects affecting the DNA “mismatch repair” pathway, which causes some tumors to generate larger numbers of mutations than others. Mismatch repair defects have been shown to predict better responses to immunotherapies, which are designed to harness the immune system against cancer .
With all the data in hand, a special panel of oncologists, pharmacologists, cancer biologists, geneticists, surgeons, radiologists, pathologists, and bioinformatics experts consulted to arrive at the right customized combination of drugs for each patient.
The panel’s findings were presented to the health care team working with each patient. The physician for each patient then had the final decision on whether to recommend the treatment regimen, balancing the panel’s suggestions with other real-world factors, such as a patient’s insurance coverage, availability of drugs, and his or her treatment preference.
Ten patients decided to stick with unmatched treatment. But 73 participants received a customized combination therapy. As no two molecular profiles were identical, the customized treatment regimens varied from person to person.
Many people received designer drugs targeting particular genetic alterations. Some also received checkpoint inhibitor immunotherapies to unleash the immune system against cancer. Four people also were treated with hormone therapies in combination with molecularly targeted drugs. In all, most regimens combined two to five drugs to target each cancer profile.
Participants were followed until their cancer progressed, they could no longer take treatment, or they died. For each person, the researchers calculated a “matching score,” roughly defined as the number of molecular alterations matched to administered drug(s), with some further calculations.
The evidence showed that those with matching scores greater than 50 percent, meaning more than half of a tumor’s identified aberrations had been targeted, were more likely to have stopped the progression of their cancers. Importantly, half of patients with the higher matching scores had prolonged stable disease (six months or longer) or a complete or partial remission. Similar results were attained in only 22 percent of those with low or no matching scores.
These encouraging results suggest that customized combinations of targeted treatments will help to advance precision oncology. However, there are still many challenges. For example, many of the combinations used in the study have not yet been safety tested. The researchers managed the potential risk of toxicities by starting patients on an initial low dose and having their physicians follow them closely while the dose was increased to a level well-tolerated by each individual patient.
And indeed, they saw no evidence that those receiving a greater proportion of “matched” drugs (i.e. those with a higher matching score) were more likely to experience adverse effects than those who took fewer drugs. So, that’s an encouraging sign.
The researchers are now enrolling patients in a new version of the I-PREDICT trial. Unlike the initial plan, patients are now being enrolled prior to receiving any treatment for a recently diagnosed aggressive, often-lethal form of cancer. The hope is that treating patients with well-matched, multi-drug treatment combinations early will yield even better results than waiting until standard treatment has failed. If correct, it would mark significant progress in building the future of precision oncology.
Reference:
[1] Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE, Miller VA, Ross JS, Benson A, Webster J, Stephens PJ, Lee JJ, Fanta PT, Lippman SM, Leyland-Jones B, Kurzrock R. Nat Med. 2019 Apr 22.
Links:
Precision Medicine in Cancer Treatment (National Cancer Institute/NIH)
Study of Molecular Profile-Related Evidence to Determine Individualized Therapy for Advanced or Poor Prognosis Cancers (I-PREDICT) (Clinicaltrials.gov)
Razelle Kurzrock (University of California, San Diego)
Jason Sicklick (University of California, San Diego)
NIH Support: National Cancer Institute
Nanodiamonds Shine in Root Canal Study
Posted on by Dr. Francis Collins
When the time comes to get relief from a dental problem, we are all glad that dentistry has come so far—much of the progress based on research supported by NIH’s National Institute of Dental and Craniofacial Research. Still, almost no one looks forward to getting a root canal. Not only can the dental procedure be uncomfortable and costly, there’s also a risk of failure due to infection or other complications. But some NIH-supported researchers have now come up with what may prove to be a dazzling strategy for reducing that risk: nanodiamonds!
That’s right, these researchers decided to add tiny diamonds—so small that millions could fit on the head of the pin—to the standard filler that dentists use to seal off a tooth’s root. Not only are these nanodiamonds extremely strong, they have unique properties that make them very attractive vehicles for delivering drugs, including antimicrobials that help fight infections of the sealed root canal.
Next Page