kidney disease
Healing Switch Links Acute Kidney Injury to Fibrosis, Suggesting Way to Protect Kidney Function
Posted on by Dr. Monica M. Bertagnolli
Healthy kidneys—part of the urinary tract—remove waste and help balance chemicals and fluids in the body. However, our kidneys have a limited ability to regenerate healthy tissue after sustaining injuries from conditions such as diabetes or high blood pressure. Injured kidneys are often left with a mix of healthy and scarred tissue, or fibrosis, which over time can compromise their function and lead to chronic kidney disease or complete kidney failure. More than one in seven adults in the U.S. are estimated to have chronic kidney disease, according to the Centers for Disease Control and Prevention, most without knowing it.
Now, a team of researchers led by Sanjeev Kumar at Cedars-Sinai Medical Center, Los Angeles, has identified a key molecular “switch” that determines whether injured kidney tissue will heal or develop those damaging scars.1 Their findings, reported in the journal Science, could lead to new and less invasive ways to detect fibrosis in the kidneys. The research could also point toward a targeted therapeutic approach that might prevent or reverse scarring to protect kidney function.
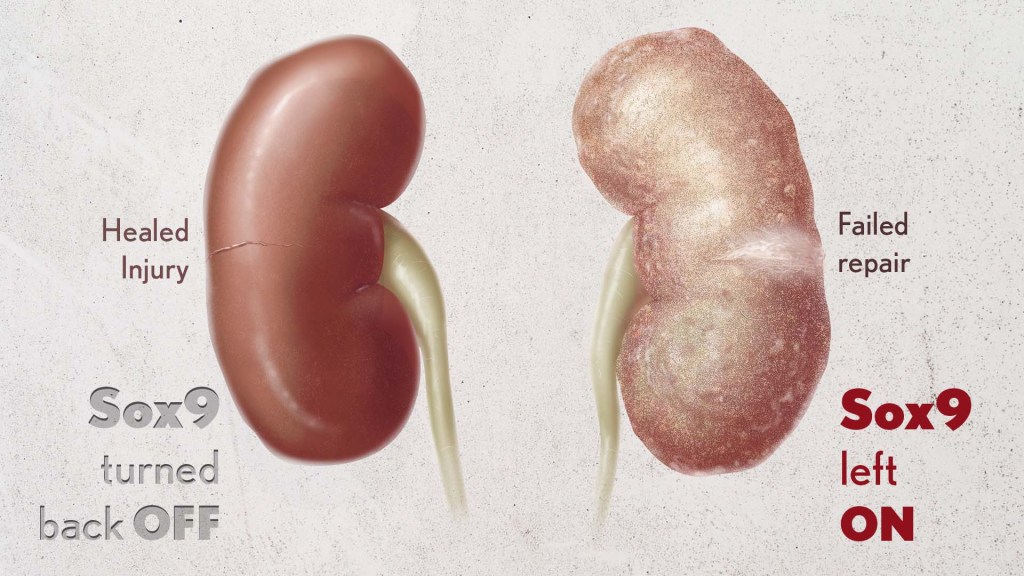
In earlier studies, the research team found that a protein called Sox9 plays an important role in switching on the repair response in kidneys after acute injury.2 In some cases, the researchers noticed that Sox9 remained active for a prolonged period of a month or more. They suspected this might be a sign of unresolved injury and repair.
By conducting studies using animal models of kidney damage, the researchers found that cells that turned Sox9 on and then back off healed without fibrosis. However, cells that failed to regenerate healthy kidney cells kept Sox9 on indefinitely, which in turn led to the production of fibrosis and scarring.
According to Kumar, Sox9 appears to act like a sensor, switching on after injury. Once restored to health, Sox9 switches back off. When healing doesn’t proceed optimally, Sox9 stays on, leading to scarring. Importantly, the researchers also found they could encourage kidneys to recover by forcing Sox9 to turn off a week after an injury, suggesting it may be a promising drug target.
The researchers also looked for evidence of this process in human patients who have received kidney transplants. They could see that, when transplanted kidneys took longer to start working, Sox9 was switched on. Those whose kidneys continued to produce Sox9 also had lower kidney function and more scarring compared to those who didn’t.
The findings suggest that the dynamics observed in animal studies may be clinically relevant in people, and that treatments targeting Sox9 might promote kidneys to heal instead of scarring. The researchers say they hope that similar studies in the future will lead to greater understanding of healing and fibrosis in other organs—including the heart, lungs, and liver—with potentially important clinical implications.
References:
[1] Aggarwal S, et al. SOX9 switch links regeneration to fibrosis at the single-cell level in mammalian kidneys. Science. DOI: 10.1126/science.add6371 (2024).
[2] Kumar S, et al. Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Reports. DOI: 10.1016/j.celrep.2015.07.034 (2015).
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Chipping Away at the Causes of Polycystic Kidney Disease
Posted on by Lawrence Tabak, D.D.S., Ph.D.
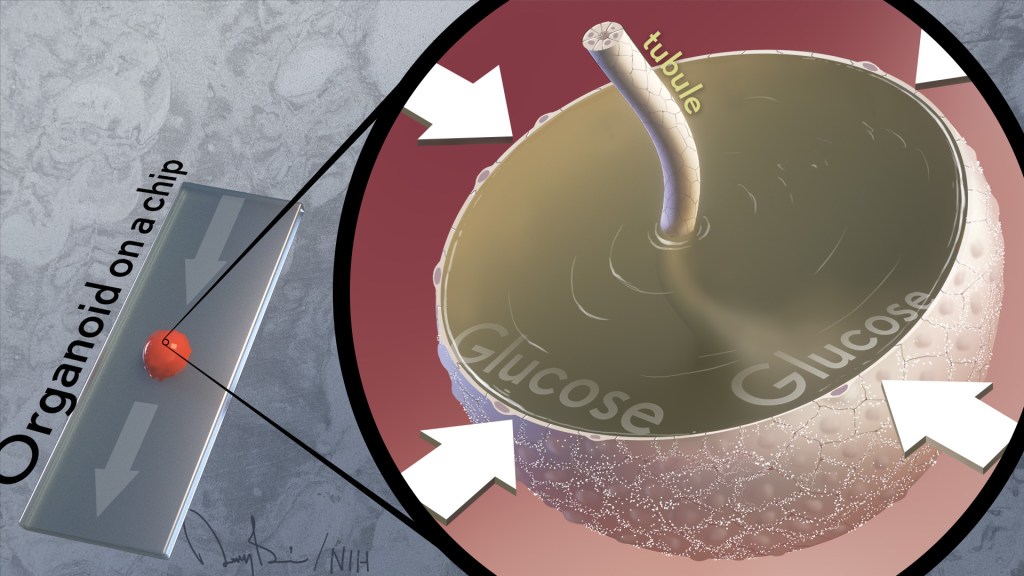
It’s often said that two is better than one. That’s true whether driving across the country, renovating a kitchen, or looking for a misplaced set of car keys. But a recent study shows this old saying also applies for modeling a kidney disease with two very complementary, cutting-edge technologies: an organoid, a living miniaturized organ grown in a laboratory dish; and an “organ-on-a-chip,” silicon chips specially engineered to mimic the 3D tissue structure and basic biology of a human body organ.
Using this one-two approach at the lab bench, the researchers modeled in just a few weeks different aspects of the fluid-filled cysts that form in polycystic kidney disease (PKD), a common cause of kidney failure. This is impossible to do in real-time in humans for a variety of technical reasons.
These powerful technologies revealed that blood glucose plays a role in causing the cysts. They also showed the cysts form via a different biological mechanism than previously thought. These new leads, if confirmed, offer a whole new way of thinking about PKD cysts, and more exciting, how to prevent or slow the disease in millions of people worldwide.
These latest findings, published in the journal Nature Communications, come from Benjamin Freedman and colleagues at the University of Washington School of Medicine, Seattle [1]. While much is known about the genetic causes of PKD, Freedman and team realized there’s much still much to learn about the basics of how cysts form in the kidney’s tiny tubes, or tubules, that help to filter toxins out of the bloodstream.
Each human kidney has millions of tubules, and in people with PKD, some of them expand gradually and abnormally to form sacs of fluid that researchers liken to water balloons. These sacs, or cysts, crowd out healthy tissue, leading over time to reduced kidney function and, in some instances, complete kidney failure.
To understand cyst formation better, Freedman’s team and others have invented methods to grow human kidney organoids, complete with a system of internal tubules. Impressively, organoids made from cells carrying mutations known to cause PKD develop cysts, just as people with these same mutations do. When suspended in fluid, the organoids also develop telltale signs of PKD even more dramatically, showing they are sensitive to changes in their environments.
At any given moment, about a quarter of all the fluids in the body pass through the kidneys, and this constant flow was missing from the organoid. That’s when Freedman and colleagues turned to their other modeling tool: a kidney-on-a-chip.
These more complex 3D models, containing living kidney cells, aim to mimic more fully the kidney and its environment. They also contain a network of microfluidic channels to replicate the natural flow of fluids in a living kidney. Combining PKD organoids with kidney-on-a-chip technology provided the best of both worlds.
Their studies found that exposing PKD organoid-on-a-chip models to a solution including water, glucose, amino acids, and other nutrients caused cysts to expand more quickly than they otherwise would. However, the cysts don’t develop from fluids that the kidneys outwardly secrete, as long thought. The new findings reveal just the opposite. The PKD cysts arise and grow as the kidney tissue works to retain most of the fluids that constantly pass through them.
They also found out why: the cysts were absorbing glucose and taking in water from the fluid passing over them, causing the cysts to expand. Although scientists had known that kidneys absorb glucose, they’d never connected this process to the formation of cysts in PKD.
In further studies, the scientists gave fluorescently labeled glucose to mice with PKD and could see that kidney cysts in the animals also took up glucose. The researchers think that the tubules are taking in fluid in the mice just as they do in the organoids.
Understanding the mechanisms of PKD can point to new ways to treat it. Indeed, the research team showed adding compounds that block the transport of glucose also prevented cyst growth. Freedman notes that glucose transport inhibitors (flozins), a class of oral drugs now used to treat diabetes, are in development for other types of kidney disease. He said the new findings suggest glucose transport inhibitors might have benefits for treating PKD, too.
There’s much more work to do. But the hope is that these new insights into PKD biology will lead to promising ways to prevent or treat this genetic condition that now threatens the lives of far too many loved ones in so many families.
This two-is-better-than-one approach is just an example of the ways in which NIH-supported efforts in tissue chips are evolving to better model human disease. That includes NIH’s National Center for Advancing Translational Science’s Tissue Chip for Drug Screening program, which is enabling promising new approaches to study human diseases affecting organ systems throughout the body.
Reference:
[1] Glucose absorption drives cystogenesis in a human organoid-on-chip model of polycystic kidney disease. Li SR, Gulieva RE, Helms L, Cruz NM, Vincent T, Fu H, Himmelfarb J, Freedman BS. Nat Commun. 2022 Dec 23;13(1):7918.
Links:
Polycystic Kidney Disease (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Your Kidneys & How They Work (NIDDK)
Freedman Lab (University of Washington, Seattle)
Tissue Chip for Drug Screening (National Center for Advancing Translational Sciences/NIH)
NIH Support: National Center for Advancing Translational Sciences; National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute
A Race-Free Approach to Diagnosing Chronic Kidney Disease
Posted on by Dr. Francis Collins
Race has a long and tortured history in America. Though great strides have been made through the work of leaders like Dr. Martin Luther King, Jr. to build an equal and just society for all, we still have more work to do, as race continues to factor into American life where it shouldn’t. A medical case in point is a common diagnostic tool for chronic kidney disease (CKD), a condition that affects one in seven American adults and causes a gradual weakening of the kidneys that, for some, will lead to renal failure.
The diagnostic tool is a medical algorithm called estimated glomerular filtration rate (eGFR). It involves getting a blood test that measures how well the kidneys filter out a common waste product from the blood and adding in other personal factors to score how well a person’s kidneys are working. Among those factors is whether a person is Black. However, race is a complicated construct that incorporates components that go well beyond biological and genetic factors to social and cultural issues. The concern is that by lumping together Black people, the algorithm lacks diagnostic precision for individuals and could contribute to racial disparities in healthcare delivery—or even runs the risk of reifying race in a way that suggests more biological significance than it deserves.
That’s why I was pleased recently to see the results of two NIH-supported studies published in The New England Journal of Medicine that suggest a way to take race out of the kidney disease equation [1, 2]. The approach involves a new equation that swaps out one blood test for another and doesn’t ask about race.
For a variety of reasons, including socioeconomic issues and access to healthcare, CKD disproportionately affects the Black community. In fact, Blacks with the condition are also almost four times more likely than whites to develop kidney failure. That’s why Blacks with CKD must visit their doctors regularly to monitor their kidney function, and often that visit involves eGFR.
The blood test used in eGFR measures creatinine, a waste product produced from muscle. For about the past 20 years, a few points have been automatically added to the score of African Americans, based on data showing that adults who identify as Black, on average, have a higher baseline level of circulating creatinine. But adjusting the score upward toward normal function runs the risk of making the kidneys seem a bit healthier than they really are and delaying life-preserving dialysis or getting on a transplant list.
A team led by Chi-yuan Hsu, University of California, San Francisco, took a closer look at the current eGFR calculations. The researchers used long-term data from the Chronic Renal Insufficiency Cohort (CRIC) Study, an NIH-supported prospective, observational study of nearly 4,000 racially and ethnically diverse patients with CKD in the U.S. The study design specified that about 40 percent of its participants should identify as Black.
To look for race-free ways to measure kidney function, the researchers randomly selected more than 1,400 of the study’s participants to undergo a procedure that allows kidney function to be measured directly instead of being estimated based on blood tests. The goal was to develop an accurate approach to estimating GFR, the rate of fluid flow through the kidneys, from blood test results that didn’t rely on race.
Their studies showed that simply omitting race from the equation would underestimate GFR in Black study participants. The best solution, they found, was to calculate eGFR based on cystatin C, a small protein that the kidneys filter from the blood, in place of the standard creatinine. Estimation of GFR using cystatin C generated similarly accurate results but without the need to factor in race.
The second NIH-supported study led by Lesley Inker, Tufts Medical Center, Boston, MA, came to similar conclusions. They set out to develop new equations without race using data from several prior studies. They then compared the accuracy of their new eGFR equations to measured GFR in a validation set of 12 other studies, including about 4,000 participants.
Their findings show that currently used equations that include race, sex, and age overestimated measured GFR in Black Americans. However, taking race out of the equation without other adjustments underestimated measured GFR in Black people. Equations including both creatinine and cystatin C, but omitting race, were more accurate. The new equations also led to smaller estimated differences between Black and non-Black study participants.
The hope is that these findings will build momentum toward widespread adoption of cystatin C for estimating GFR. Already, a national task force has recommended immediate implementation of a new diagnostic equation that eliminates race and called for national efforts to increase the routine and timely measurement of cystatin C [3]. This will require a sea change in the standard measurements of blood chemistries in clinical and hospital labs—where creatinine is routinely measured, but cystatin C is not. As these findings are implemented into routine clinical care, let’s hope they’ll reduce health disparities by leading to more accurate and timely diagnosis, supporting the goals of precision health and encouraging treatment of CKD for all people, regardless of their race.
References:
[1] Race, genetic ancestry, and estimating kidney function in CKD. Hsu CY, Yang W, Parikh RV, Anderson AH, Chen TK, Cohen DL, He J, Mohanty MJ, Lash JP, Mills KT, Muiru AN, Parsa A, Saunders MR, Shafi T, Townsend RR, Waikar SS, Wang J, Wolf M, Tan TC, Feldman HI, Go AS; CRIC Study Investigators. N Engl J Med. 2021 Sep 23.
[2] New creatinine- and cystatin C-based equations to estimate GFR without race. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH,Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration. N Engl J Med. 2021 Sep 23.
[3] A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, St Peter WL, Warfield C, Powe NR. Am J Kidney Dis. 2021 Sep 22:S0272-6386(21)00828-3.
Links:
Chronic Kidney Disease (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Explaining Your Kidney Test Results: A Tool for Clinical Use (NIDDK)
Chronic Renal Insufficiency Cohort Study
Chi-yuan Hsu (University of California, San Francisco)
Lesley Inker (Tufts Medical Center, Boston)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Building Nanoparticles for Kidney Disease
Posted on by Dr. Francis Collins

Great things sometimes come in small packages. That’s certainly true in the lab of Eun Ji Chung at the University of Southern California, Los Angeles. Chung and her team each day wrap their brains around bioengineering 3-D nanoparticles, molecular constructs that measure just a few billionths of a meter.
Chung recently received an NIH Director’s 2018 New Innovator Award to bring the precision of nanomedicine to autosomal dominant polycystic kidney disease (ADPKD), a relatively common inherited disorder that affects about 600,000 Americans and 12 million people worldwide.
By age 60, about half of those battling ADPKD will have kidney failure, requiring dialysis or a kidney transplant to stay alive. For people with ADPKD, a dominantly inherited gene mutation causes clusters of fluid-filled cysts to form in the kidneys that grow larger over time. The cysts can grow very large and displace normal kidney tissue, progressively impairing function.
For Chung, the goal is to design nanoparticles of the right size and configuration to deliver therapeutics to the kidneys in safe, effective amounts. Our kidneys constantly filter blood, clearing out wastes that are removed via urine. So, Chung and her team will exploit the fact that most molecules in the bloodstream measuring less than 10 nanometers in diameter enter the kidneys, where they are gradually processed and eliminated from the body. This process will give nanoparticles time to bind there and release any therapeutic molecules they may be carrying directly to the cysts that cluster on the kidneys of people with ADPKD.
Chung’s research couldn’t be more timely. Though ADPKD isn’t curable right now, the Food and Drug Administration (FDA) last year approved Jynarque™ (tolvaptan), the first treatment in the United States to slow the decline in kidney function in ADPKD patients, based on tests of the rate of kidney filtration. Other approved drugs, such as metformin and rapamycin, have shown potential for repurposing to treat people with ADPKD. So, getting these and other potentially life-saving drugs directly to the kidneys, while minimizing the risk of serious side effects in the liver and elsewhere in the body, will be key.
Most FDA-approved nanoparticle therapies are administered intravenously, often for treatment of cancer. Because ADPKD is chronic and treatment can last for decades, Chung wants to develop an easy-to-take pill to get these nanoparticles into the kidneys.
But oral administration raises its own set of difficulties. The nanoparticles must get from the stomach and the rest of the gastrointestinal tract to the bloodstream. And then if nanoparticles exceed 10 nanometers in diameter, the body typically routes them to the liver for clearance, rather than the kidneys.
While Chung brainstorms strategies for oral administration, she’s also considering developing a smart bandage to allow the nanoparticles to pass readily through the skin and, eventually, into the bloodstream. It would be something similar to the wearable skin patch already featured on the blog.
In the meantime, Chung continues to optimize the size, shape, and surface charge of her nanoparticles. Right now, they have components to target the kidneys, provide a visual signal for tracking, enhance the nanoparticle’s lifespan, and carry a therapeutic molecule. Because positively charged molecules are preferentially attracted to the kidney, Chung has also spent untold hours adjusting the charge on her nanoparticles.
But through all the hard work, Chung and her team continue to prove that great things may one day come in very small packages. And that could ultimately prove to be a long-awaited gift for the millions of people living with ADPKD.
Links:
Polycystic Kidney Disease (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Video: Faculty Profile – Eun Ji Chung (University of Southern California, Los Angeles)
Chung Laboratory (USC)
Chung Project Information (NIH RePORTER)
NIH Director’s New Innovator Award (Common Fund)
NIH Support: Common Fund; National Institute of Diabetes and Digestive and Kidney Diseases
Using Frogs to Tackle Kidney Problems
Posted on by Dr. Francis Collins
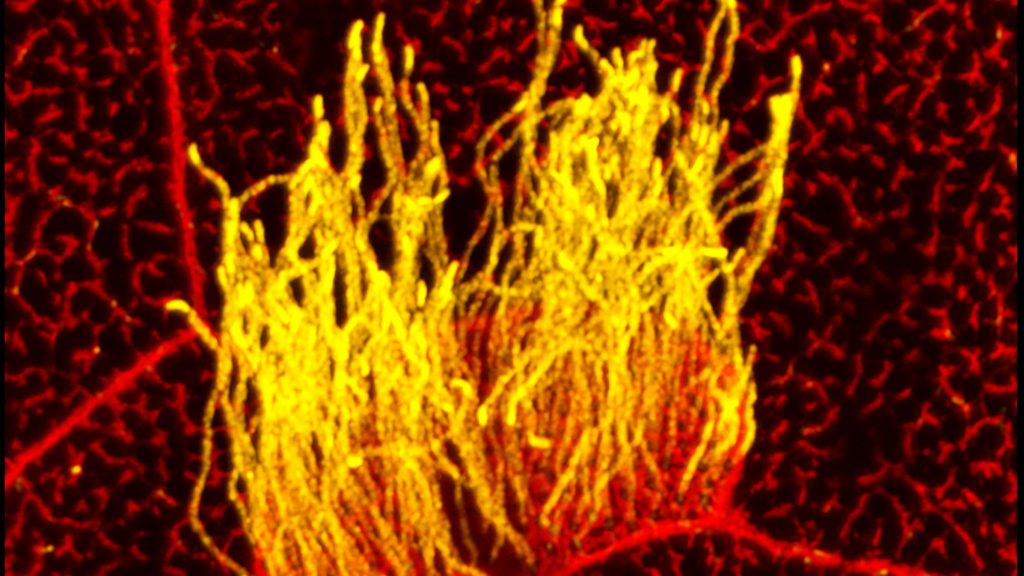
Many human cells are adorned with hair-like projections called cilia. Scientists now realize that these specialized structures play many important roles throughout the body, including directing or sensing various signals such as fluid flow. Their improper function has been linked to a wide range of health conditions, such as kidney disease, scoliosis, and obesity.
Studying cilia in people can be pretty challenging. It’s less tricky in a commonly used model organism: Xenopus laevis, or the African clawed frog. This image highlights a healthy patch of motile cilia (yellow) on embryonic skin cells (red) of Xenopus laevis. The cilia found in humans and all other vertebrates are built from essentially the same elongated structures known as microtubules. That’s why researchers can learn a lot about human cilia by studying frogs.
Pursuing Precision Medicine for Chronic Kidney Disease
Posted on by Dr. Francis Collins
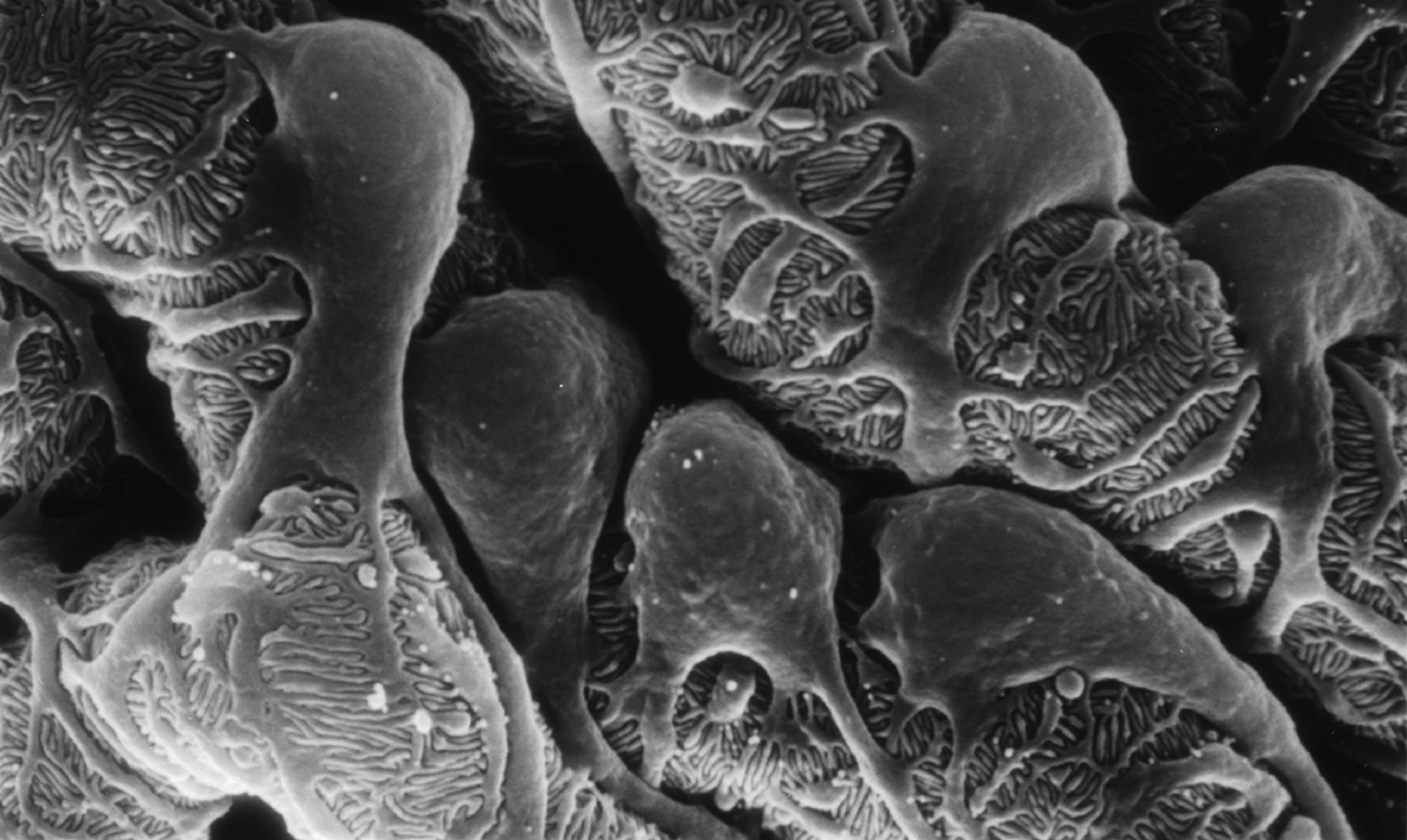
Caption: Scanning electron micrograph showing a part of one of the kidney’s glomerular filters, which are damaged in people with chronic kidney disease (CKD). The cells with the lacy cytoplasmic extensions are called podocytes.
Credit: Kretzler Lab, University of Michigan Health System, Ann Arbor
Every day, our kidneys filter more than 30 gallons of blood to allow excretion of molecules that can harm us if they build up as waste. But, for more than 20 million Americans and a growing number of people around the world, this important function is compromised by chronic kidney disease (CKD) [1]. Some CKD patients are at high risk of progressing to actual kidney failure, treatable only by dialysis or kidney transplants, while others remain generally healthy with stable kidney function for many years with minimal treatment.
The dilemma is that, even when CKD is diagnosed early, there’s been no good way to predict which individuals are at high risk for rapid progression. Those individuals would potentially benefit from more intensive measures to slow or prevent kidney failure, such as drug regimens that tightly control blood pressure and/or blood glucose. So, I’m pleased to report that NIH-funded researchers have made some progress toward developing more precise strategies for identifying individuals at high risk for kidney failure. In recent findings published in Science Translational Medicine [2], an international research team has identified a protein, easily detectable in urine, which appears to serve as an early warning sign of CKD progression.
A wide range of conditions, from diabetes to hypertension to the autoimmune disease lupus, can contribute to the gradual loss of kidney function seen in people with CKD. But research suggests that once kidney damage reaches a critical threshold, it veers off to follow a common downhill course, driven by shared cell signaling pathways and almost independent of the conditions causing it. If there was an easy, reliable way to determine when a CKD patient’s kidneys are approaching this threshold, it could open the door to better strategies for protecting them from kidney failure.
With this need in mind, a team, led by Matthias Kretzler and Wenjun Ju of the University of Michigan, began analyzing gene activity in kidney biopsy samples donated by 164 CKD patients and stored in the European Renal cDNA Bank. Specifically, the researchers looked for patterns of gene activity that corresponded with the patients’ estimated glomerular filtration rates, an indicator of renal function frequently calculated as part of a routine blood workup. Their first pass produced a list of 72 genes that displayed varying levels of activity that corresponded to differences in the patients’ estimated glomerular filtration rates. Importantly, the activity of many of those genes is also increased in cell signaling pathways thought to drive CKD progression.
Further study in two more groups of CKD patients, one from the United States and another from Europe, whittled the list down to three genes that best predicted kidney function. The researchers then zeroed in on the gene that codes for epidermal growth factor (EGF), a protein that, within the kidney, seems to be produced specifically in tubules, which are key components of the waste filtration system. Because EGF appears to enhance tubular repair after injury, researchers had a hunch that it might serve as a positive biomarker of tubular function that could be combined with existing tests of glomerular filtration to detect progression of CKD at an earlier stage.
In groups of CKD patients from the United States and China, the researchers went on to find that the amount of EGF in the urine provides an accurate measure of the protein’s activity in the kidney, making it a promising candidate for a simple urine test. In fact, CKD patients with low levels of EGF in their urine were four times more likely than those with higher EGF levels to have their kidney function worsen within a few years.
These lines of evidence suggest that, if these findings are replicated in additional studies, it may be possible to develop a simple EGF urine test to help identify which individuals with CKD would benefit the most from aggressive disease management and clinical follow-up. Researchers also plan to explore the possibility that such a urine test might prove useful in the early diagnosis of CKD, before there are any other indications of kidney disease. These are very promising new findings, but much remains to be done before we can think of applying these results as standard of care in the clinic. For example, the EGF work needs to be replicated in larger groups of CKD patients, as well as CKD patients with diabetes.
Beyond their implications for CKD, these results demonstrate the power of identifying new biologically important indicators directly from patients and then testing them in large, diverse cohorts of people. I look forward to the day when these sorts of studies will become possible on an even larger scale through our U.S. Precision Medicine Initiative Cohort.
References:
[1] National Chronic Kidney Disease Fact Sheet, 2014. Centers for Disease Control and Prevention.
[2] Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PX, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M; ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium. Sci Transl Med. 2015 Dec 2;7(316):316ra193.
Links:
Chronic Kidney Disease: What Does it Mean to Me? (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Personalized Molecular Nephrology Research Laboratory (University of Michigan)
C-Probe (University of Michigan)
Precision Medicine Initiative Cohort Program (NIH)
NIH Support: National Center for Advancing Translational Sciences; National Institute of Diabetes and Digestive and Kidney Diseases