13 Search Results for "regeneration"
Snapshots of Life: Wired for Nerve Regeneration
Posted on by Dr. Francis Collins
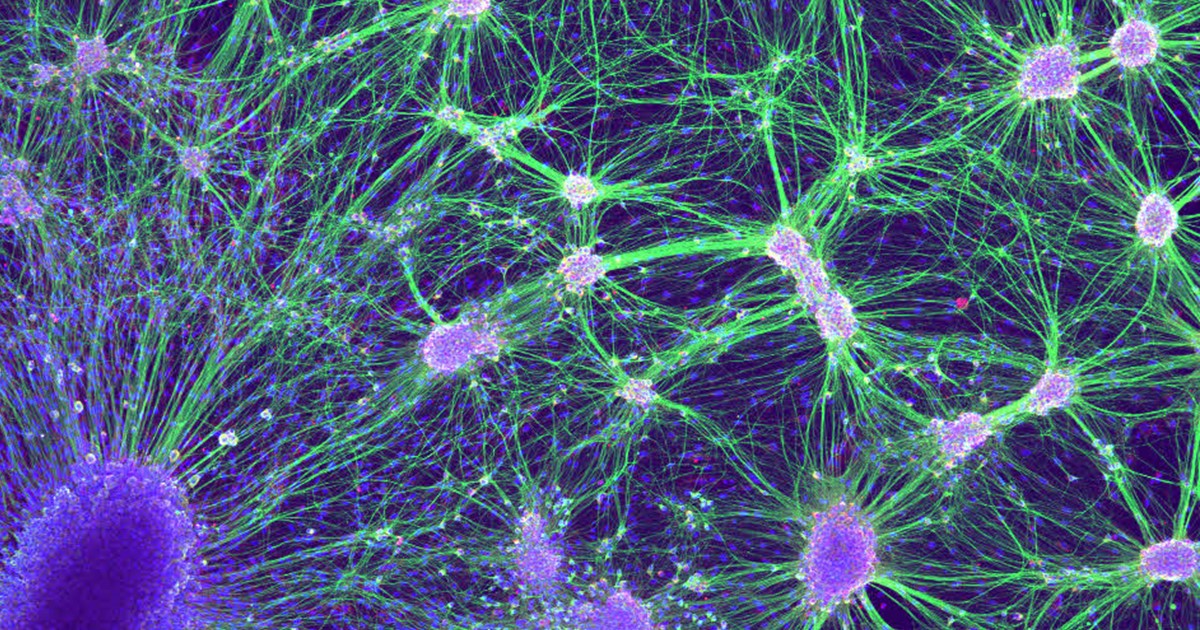
Credit: Laura Struzyna, Cullen Laboratory, Perelman School of Medicine, University of Pennsylvania, Philadelphia
Getting nerve cells to grow in the lab can be a challenge. But when it works, the result can be a thing of beauty for both science and art. What you see growing in the Petri dish shown above are nerve cells from an embryonic rat. On the bottom left is a dorsal root ganglion (dark purple), which is a cluster of sensory nerve bodies normally found just outside the spinal cord. To the right are the nuclei (light purple) and axons (green) of motor neurons, which are the nerve cells involved in forming key signaling networks.
Laura Struzyna, a graduate student in the lab of NIH grantee D. Kacy Cullen at the University of Pennsylvania’s Perelman School of Medicine, Philadelphia, is using laboratory-grown nerve cells in her efforts to learn how to bioengineer nerve grafts. The hope is this work will one day lead to grafts that can be used to treat people whose nerves have been damaged by car accidents or other traumatic injuries.
Healing Switch Links Acute Kidney Injury to Fibrosis, Suggesting Way to Protect Kidney Function
Posted on by Dr. Monica M. Bertagnolli
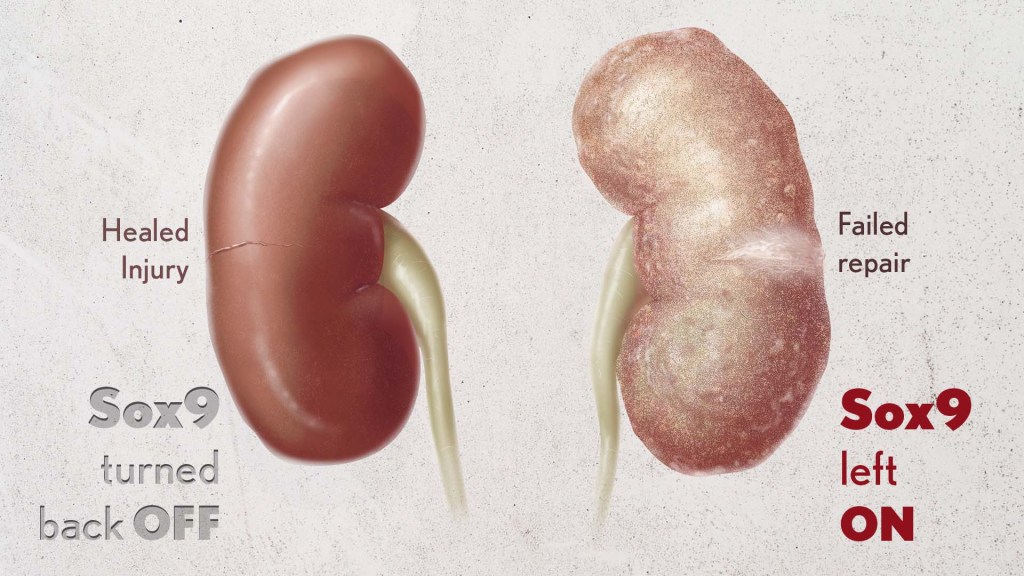
Healthy kidneys—part of the urinary tract—remove waste and help balance chemicals and fluids in the body. However, our kidneys have a limited ability to regenerate healthy tissue after sustaining injuries from conditions such as diabetes or high blood pressure. Injured kidneys are often left with a mix of healthy and scarred tissue, or fibrosis, which over time can compromise their function and lead to chronic kidney disease or complete kidney failure. More than one in seven adults in the U.S. are estimated to have chronic kidney disease, according to the Centers for Disease Control and Prevention, most without knowing it.
Now, a team of researchers led by Sanjeev Kumar at Cedars-Sinai Medical Center, Los Angeles, has identified a key molecular “switch” that determines whether injured kidney tissue will heal or develop those damaging scars.1 Their findings, reported in the journal Science, could lead to new and less invasive ways to detect fibrosis in the kidneys. The research could also point toward a targeted therapeutic approach that might prevent or reverse scarring to protect kidney function.
In earlier studies, the research team found that a protein called Sox9 plays an important role in switching on the repair response in kidneys after acute injury.2 In some cases, the researchers noticed that Sox9 remained active for a prolonged period of a month or more. They suspected this might be a sign of unresolved injury and repair.
By conducting studies using animal models of kidney damage, the researchers found that cells that turned Sox9 on and then back off healed without fibrosis. However, cells that failed to regenerate healthy kidney cells kept Sox9 on indefinitely, which in turn led to the production of fibrosis and scarring.
According to Kumar, Sox9 appears to act like a sensor, switching on after injury. Once restored to health, Sox9 switches back off. When healing doesn’t proceed optimally, Sox9 stays on, leading to scarring. Importantly, the researchers also found they could encourage kidneys to recover by forcing Sox9 to turn off a week after an injury, suggesting it may be a promising drug target.
The researchers also looked for evidence of this process in human patients who have received kidney transplants. They could see that, when transplanted kidneys took longer to start working, Sox9 was switched on. Those whose kidneys continued to produce Sox9 also had lower kidney function and more scarring compared to those who didn’t.
The findings suggest that the dynamics observed in animal studies may be clinically relevant in people, and that treatments targeting Sox9 might promote kidneys to heal instead of scarring. The researchers say they hope that similar studies in the future will lead to greater understanding of healing and fibrosis in other organs—including the heart, lungs, and liver—with potentially important clinical implications.
References:
[1] Aggarwal S, et al. SOX9 switch links regeneration to fibrosis at the single-cell level in mammalian kidneys. Science. DOI: 10.1126/science.add6371 (2024).
[2] Kumar S, et al. Sox9 Activation Highlights a Cellular Pathway of Renal Repair in the Acutely Injured Mammalian Kidney. Cell Reports. DOI: 10.1016/j.celrep.2015.07.034 (2015).
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
How to Heal Skin Without the Scars
Posted on by Dr. Francis Collins

Most of us can point to a few unwanted scars on our bodies. Every scar tells a story, but people are spending billions of dollars each year trying to hide or get rid of them [1]. What if there was a way to get the wounds on our skin to heal without scarring in the first place?
In a recent paper in the journal Science, a team of NIH-supported researchers has taken an important step in this direction. Working with mice, the researchers deciphered some of the key chemical and physical signals that cause certain skin cells to form tough, fibrous scars while healing a wound [2]. They also discovered how to reprogram them with a topical treatment and respond to injuries more like fetal skin cells, which can patch up wounds in full, regrowing hair, glands, and accessory structures of the skin, and all without leaving a mark.
Of course, mice are not humans. Follow-up research is underway to replicate these findings in larger mammals with skin that’s tighter and more akin to ours. But if the preclinical data hold up, the researchers say they can test in future human clinical trials the anti-scarring drug used in the latest study, which has been commercially available for two decades to treat blood vessel disorders in the eye.
The work comes from Michael Longaker, Shamik Mascharak, and colleagues, Stanford Medicine, Palo Alto, CA. But, to be more precise, the work began with a research project that Longaker was given back in 1987, while a post doc in the lab of Michael Harrison, University of California, San Francisco.
Harrison, a surgeon then performing groundbreaking prenatal surgery, noticed that babies born after undergoing surgery in the womb healed from their surgeries without any scarring. He asked his postdoc to find out why, and Longaker has been trying to answer that question and understand scar formation ever since.
Longaker and his Stanford colleague Geoffrey Gurtner suspected that the difference between healing inside and outside the womb had something to do with tension. Inside the womb, the skin of the unborn is bathed in fluid and develops in a soft, tension-free state. Outside the womb, human skin is exposed to continuous environmental stresses and must continuously remodel and grow to remain viable, which creates a high level of skin tension.
Following up on Longaker and Gurtner’s suspicion, Mascharak found in a series of mouse experiments that a particular class of fibroblast, a type of cell in skin and other connective tissues, activates a gene called Engrailed-1 during scar formation [3]. To see if mechanical stress played a role in this process, Mascharak and team grew mouse fibroblast cells on either a soft, stress-free gel or on a stiff plastic dish that produced mechanical strain. Importantly, they also tried growing the fibroblasts on the same strain-inducing plastic, but in the presence of a chemical that blocked the mechanical-strain signal.
Their studies showed that fibroblasts grown on the tension-free gel didn’t activate the scar-associated genetic program, unlike fibroblasts growing on the stress-inducing plastic. With the chemical that blocked the cells’ ability to sense the mechanical strain, Engrailed-1 didn’t get switched on either.
They also showed the opposite. When tension was applied to healing surgical incisions in mice, it led to an increase in the number of those fibroblast cells expressing Engrailed-1 and thicker scars.
The researchers went on to make another critical finding. The mechanical stress of a fresh injury turns on a genetic program that leads to scar formation, and that program gets switched on through another protein called Yes-associated protein (YAP). When they blocked this protein with an existing eye drug called verteporfin, skin healed more slowly but without any hint of a scar.
It’s worth noting that scars aren’t just a cosmetic issue. Scars differ from unwounded skin in many ways. They lack hair follicles, glands that produce oil and sweat, and nerves for sensing pain or pressure. Because the fibers that make up scar tissue run parallel to each other instead of being more intricately interwoven, scars also lack the flexibility and strength of healthy skin.
These new findings therefore suggest it may one day be possible to allow wounds to heal without compromising the integrity of the skin. The findings also may have implications for many other medical afflictions that involve scarring, such as liver and lung fibrosis, burns, scleroderma, and scarring of heart tissue after a heart attack. That’s also quite a testament to sticking with a good postdoc project, wherever it may lead. One day, it may even improve public health!
References:
[1] Human skin wounds: A major and snowballing threat to public health and the economy. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Wound Repair Regen. 2009 Nov-Dec;17(6):763-771.
[2] Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring.
Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, Shen AH, Januszyk M, Kwon SH, Wernig G, Wan DC, Lorenz HP, Gurtner GC, Longaker MT. Science. 2021 Apr 23;372(6540):eaba2374.
[3] Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Science. 2015 Apr 17;348(6232):aaa2151.
Links:
Skin Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases/NIH)
Scleroderma (NIAMS)
Michael Longaker (Stanford Medicine, Palo Alto, CA)
Geoffrey Gurtner (Stanford Medicine)
NIH Support: National Institute of General Medical Sciences; National Institute of Dental and Craniofacial Research
Skin Cells Can Be Reprogrammed In Vivo
Posted on by Dr. Francis Collins

Thousands of Americans are rushed to the hospital each day with traumatic injuries. Daniel Gallego-Perez hopes that small chips similar to the one that he’s touching with a metal stylus in this photo will one day be a part of their recovery process.
The chip, about one square centimeter in size, includes an array of tiny channels with the potential to regenerate damaged tissue in people. Gallego-Perez, a researcher at The Ohio State University Colleges of Medicine and Engineering, Columbus, has received a 2018 NIH Director’s New Innovator Award to develop the chip to reprogram skin and other cells to become other types of tissue needed for healing. The reprogrammed cells then could regenerate and restore injured neural or vascular tissue right where it’s needed.
Gallego-Perez and his Ohio State colleagues wondered if it was possible to engineer a device placed on the skin that’s capable of delivering reprogramming factors directly into cells, eliminating the need for the viral delivery vectors now used in such work. While such a goal might sound futuristic, Gallego-Perez and colleagues offered proof-of-principle last year in Nature Nanotechnology that such a chip can reprogram skin cells in mice. [1]
Here’s how it works: First, the chip’s channels are loaded with specific reprogramming factors, including DNA or proteins, and then the chip is placed on the skin. A small electrical current zaps the chip’s channels, driving reprogramming factors through cell membranes and into cells. The process, called tissue nanotransfection (TNT), is finished in milliseconds.
To see if the chips could help heal injuries, researchers used them to reprogram skin cells into vascular cells in mice. Not only did the technology regenerate blood vessels and restore blood flow to injured legs, the animals regained use of those limbs within two weeks of treatment.
The researchers then went on to show that they could use the chips to reprogram mouse skin cells into neural tissue. When proteins secreted by those reprogrammed skin cells were injected into mice with brain injuries, it helped them recover.
In the newly funded work, Gallego-Perez wants to take the approach one step further. His team will use the chip to reprogram harder-to-reach tissues within the body, including peripheral nerves and the brain. The hope is that the device will reprogram cells surrounding an injury, even including scar tissue, and “repurpose” them to encourage nerve repair and regeneration. Such an approach may help people who’ve suffered a stroke or traumatic nerve injury.
If all goes well, this TNT method could one day fill an important niche in emergency medicine. Gallego-Perez’s work is also a fine example of just one of the many amazing ideas now being pursued in the emerging field of regenerative medicine.
Reference:
[1] Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Gallego-Perez D, Pal D, Ghatak S, Malkoc V, Higuita-Castro N, Gnyawali S, Chang L, Liao WC, Shi J, Sinha M, Singh K, Steen E, Sunyecz A, Stewart R, Moore J, Ziebro T, Northcutt RG, Homsy M, Bertani P, Lu W, Roy S, Khanna S, Rink C, Sundaresan VB, Otero JJ, Lee LJ, Sen CK. Nat Nanotechnol. 2017 Oct;12(10):974-979.
Links:
Stroke Information (National Institute of Neurological Disorders and Stroke/NIH)
Burns and Traumatic Injury (NIH)
Peripheral Neuropathy (National Institute of Neurological Disorders and Stroke/NIH)
Video: Breakthrough Device Heals Organs with a Single Touch (YouTube)
Gallego-Perez Lab (The Ohio State University College of Medicine, Columbus)
Gallego-Perez Project Information (NIH RePORTER)
NIH Support: Common Fund; National Institute of Neurological Disorders and Stroke
Snapshots of Life: Coming Face to Face with Development
Posted on by Dr. Francis Collins
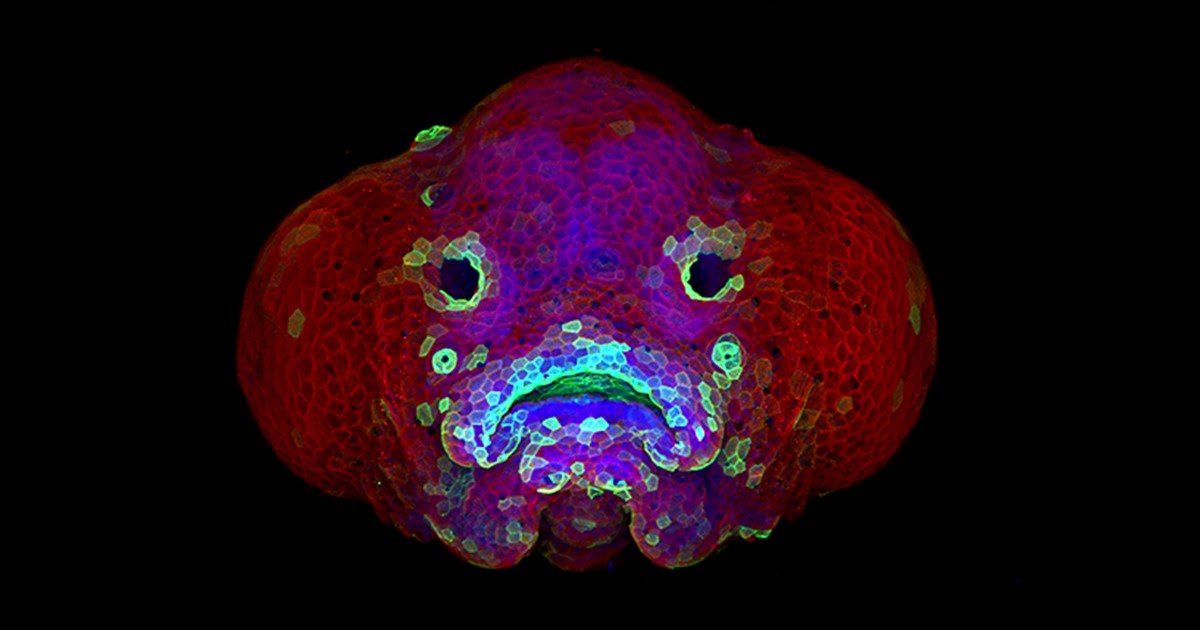
Credit: Oscar Ruiz and George Eisenhoffer, University of Texas MD Anderson Cancer Center, Houston
Zebrafish (Danio rerio) is a favorite model for studying development, in part because its transparent embryos make it possible to produce an ever-growing array of amazingly informative images. For one recent example, check out this Federation of American Societies for Experimental Biology’s 2016 BioArt winner, which shows the developing face of a 6-day-old zebrafish larva.
Yes, those downturned “lips” are indeed cells that will go on to become the fish’s mouth. But all is not quite what it appears: the two dark circles that look like eyes are actually developing nostrils. Both the nostrils and mouth express high levels of F-actin (green), a structural protein that helps orchestrate cell movement. Meanwhile, the two bulging areas on either side of the fish’s head, which are destined to become eyes and skin, express keratin (red).
Oscar Ruiz, who works in the lab of George Eisenhoffer at The University of Texas MD Anderson Cancer Center, Houston, used a confocal microscope to create this image. What was most innovative about his work was not the microscope itself, but how he prepared the sample for imaging. With traditional methods, researchers can only image the faces of zebrafish larvae from the side or the bottom. However, the Eisenhoffer lab has devised a new method of preparing fish larvae that makes it possible to image their faces head-on. This has enabled the team to visualize facial development at much higher resolution than was previously possible.
Regenerative Medicine: New Clue from Fish about Healing Spinal Cord Injuries
Posted on by Dr. Francis Collins
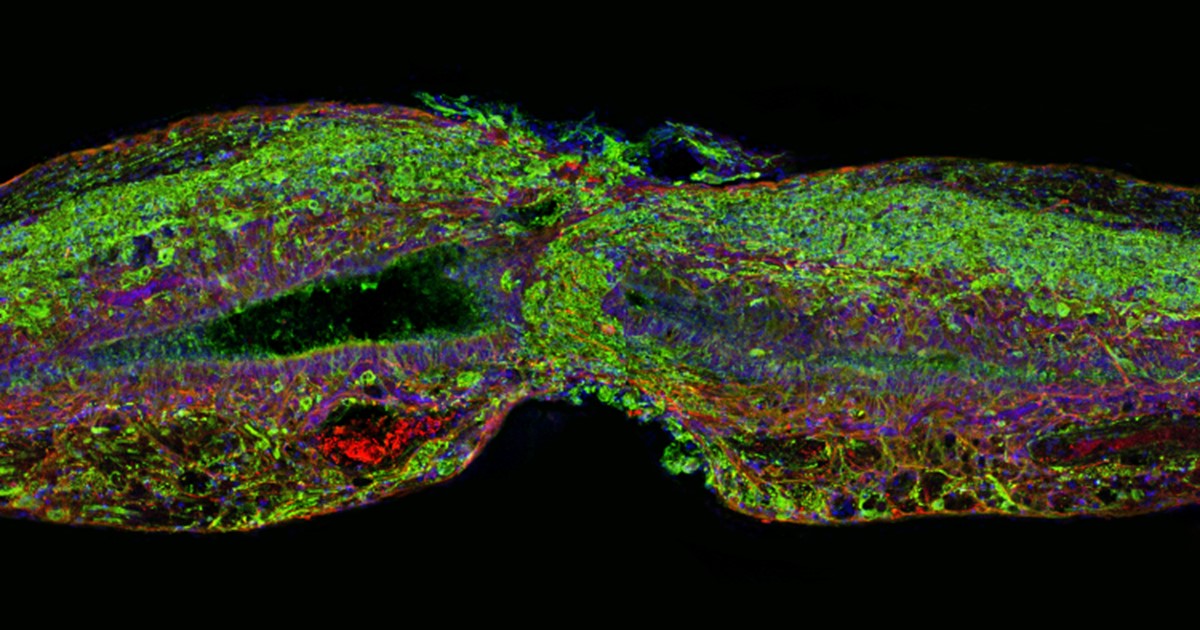
Caption: Tissue section of zebrafish spinal cord regenerating after injury. Glial cells (red) cross the gap between the severed ends first. Neuronal cells (green) soon follow. Cell nuclei are stained blue and purple.
Credit: Mayssa Mokalled and Kenneth Poss, Duke University, Durham, NC
Certain organisms have remarkable abilities to achieve self-healing, and a fascinating example is the zebrafish (Danio rerio), a species of tropical freshwater fish that’s an increasingly popular model organism for biological research. When the fish’s spinal cord is severed, something remarkable happens that doesn’t occur in humans: supportive cells in the nervous system bridge the gap, allowing new nerve tissue to restore the spinal cord to full function within weeks.
Pretty incredible, but how does this occur? NIH-funded researchers have just found an important clue. They’ve discovered that the zebrafish’s damaged cells secrete a molecule known as connective tissue growth factor a (CTGFa) that is essential in regenerating its severed spinal cord. What’s particularly encouraging to those looking for ways to help the 12,000 Americans who suffer spinal cord injuries each year is that humans also produce a form of CTGF. In fact, the researchers found that applying human CTGF near the injured site even accelerated the regenerative process in zebrafish. While this growth factor by itself is unlikely to produce significant spinal cord regeneration in human patients, the findings do offer a promising lead for researchers pursuing the next generation of regenerative therapies.
Creative Minds: Can Salamanders Show Us How to Regrow Limbs?
Posted on by Dr. Francis Collins

Jessica Whited /Credit: LightChaser Photography
Jessica Whited enjoys spending time with her 6-year-old twin boys, reading them stories, and letting their imaginations roam. One thing Whited doesn’t need to feed their curiosity about, however, is salamanders—they hear about those from Mom almost every day. Whited already has about 1,000 rare axolotl salamanders in her lab at Harvard University and Brigham and Women’s Hospital, Cambridge, MA. But caring for the 9-inch amphibians, which originate from the lakes and canals underlying Mexico City, certainly isn’t child’s play. Axolotls are entirely aquatic–their name translates to “water monster”; they like to bite each other; and they take 9 months to reach adulthood.
Like many other species of salamander, the axolotl (Ambystoma mexicanum) possesses a remarkable, almost magical, ability to grow back lost or damaged limbs. Whited’s interest in this power of limb regeneration earned her a 2015 NIH Director’s New Innovator Award. Her goal is to discover how the limbs of these salamanders know exactly where they’ve been injured and start regrowing from precisely that point, while at the same time forging vital new nerve connections to the brain. Ultimately, she hopes her work will help develop strategies to explore the possibility of “awakening” this regenerative ability in humans with injured or severed limbs.
Snapshots of Life: Fish Awash in Color
Posted on by Dr. Francis Collins

If this image makes you think of a modern art, you’re not alone. But what you’re actually seeing are hundreds of live cells from a tiny bit (0.0003348 square inches) of skin on the tail fin of a genetically engineered adult zebrafish. Zebrafish are normally found in tropical freshwater and are a favorite research model to study vertebrate development and tissue regeneration. The cells have been labeled with a cool, new fluorescent imaging tool called Skinbow. It uniquely color codes cells by getting them to express genes encoding red, green, and blue fluorescent proteins at levels that are randomly determined. The different ratios of these colorful proteins mix to give each cell a distinctive hue when imaged under a microscope. Here, you can see more than 70 detectable Skinbow colors that make individual cells as visually distinct from one another as jellybeans in a jar.
Skinbow is the creation of NIH-supported scientists Chen-Hui Chen and Kenneth Poss at Duke University, Durham, NC, with imaging computational help from collaborators Stefano Di Talia and Alberto Puliafito. As reported recently in the journal Developmental Cell [1], Skinbow’s distinctive spectrum of color occurs primarily in the outermost part of the skin in a layer of non-dividing epithelial cells. Using Skinbow, Poss and colleagues tracked these epithelial cells, individually and as a group, over their entire 2 to 3 week lifespans in the zebrafish. This gave them an unprecedented opportunity to track the cellular dynamics of wound healing or the regeneration of lost tissue over time. While Skinbow only works in zebrafish for now, in theory, it could be adapted to mice and maybe even humans to study skin and possibly other organs.
Next Page

