Generic
Visit the New NIH Virtual Tour
Posted on by Lawrence Tabak, D.D.S., Ph.D.
Happy Fourth of July! Before everyone heads out to celebrate the holiday with their family and friends, I want to share this brief video with you. It’s an introduction to the brand-new NIH Virtual Tour that’s now available on our website. When time permits, I encourage everyone to take the full tour of our Bethesda, MD, main campus and explore this great institution of science, technological innovation, and, above all, hope.
Among the virtual tour’s many features is an interactive, aerial map of the 32 buildings on our Bethesda campus. By clicking on a highlighted building, you can explore an impressive multimedia gallery of photos, video clips, and other resources. The tour will allow you to learn more about NIH and the ways in which we help people live longer and healthier lives.
You also can learn more about NIH’s 27 Institutes and Centers, including the NIH Clinical Center and 20 other in-depth tour stops—from research labs to patient rooms—and hear directly from some of our impressive researchers, leaders, and patients. For example, you can learn about chronic pain research from a lab in the NIH Clinical Center or see the largest zebrafish facility in the world, housed in Building 6.
What I like most about the virtual tour is that it captures what makes NIH so special—the many amazing people who collaborate every day to discover ways to solve seemingly intractable research problems. I admire their commitment to follow the science wherever it may lead.
In fact, from its humble beginnings in a one-room laboratory in 1887, NIH has become the world’s largest funder of medical research, whether that’s mobilizing to combat a deadly pandemic or strategizing to help people with a rare disorder find answers.
Not only does NIH conduct groundbreaking research in its own labs and clinics, it also supports much of the medical research conducted at universities and institutions in your states and local communities. Whether in Bethesda or beyond the Beltway, this national research effort will continue to yield the needed understanding to turn discovery into better health, helping more people to flourish and lead fully productive lives, now and in the generations to come.
That’s certainly something we can all celebrate this holiday, the 247th birthday of our great nation that I’m so honored to serve. Have a great, but safe, Fourth of July, and I’ll see you back here soon to share another blog post and another story of NIH-supported research progress.
Links:
Virtual Tour (NIH)
Visitor Information (NIH)
Expanding Menopause Research to Advance the Health of All Women
Posted on by Janine Austin Clayton, M.D., FARVO, NIH Office of Research on Women’s Health
Since 2017, NIH’s Office of Research on Women’s Health (ORWH) has hosted the Vivian W. Pinn Symposium during National Women’s Health Week (NWHW) in May. This event honors the first full-time director of the office, Dr. Vivian W. Pinn, and serves as a critical forum for experts across sectors to communicate and collaborate for the advancement of women’s health.
This week marks the beginning of the 2023 NWHW, and on May 16, ORWH will host the 7th Annual Vivian W. Pinn Symposium. It’s titled: Menopause and Optimizing Midlife Health of Women.
Topics to be discussed include: the menopausal transition (also known as perimenopause), the accumulation of morbidity after menopause, menopause in special populations, the influence of social determinants of health on the experience of menopause, the use of menopausal hormone therapy (MHT), and interventions to promote healthy aging.
This year, JoAnn Manson, Harvard Medical School, Cambridge, MA, will deliver the keynote speech, titled “Menopausal Hormone Therapy: 30 Years of Lessons from the Women’s Health Initiative.” I encourage everyone with an interest in women’s health to register for the event.
In 1992, NIH’s National Heart, Lung, and Blood Institute launched the Women’s Health Initiative (WHI), seeking to improve the health of women through research on prevention of serious health conditions in postmenopausal women. Over three decades later, WHI remains an extraordinary example of centering research around the health needs of women, and WHI research results “definitively established that menopausal hormone therapy should not be used to prevent heart disease, stroke, and other chronic diseases.” These results were practice-changing and led to a dramatic decline in the use of MHT.
Menopause is a natural and irreversible life course stage marked by the cessation of menstrual cycling for 12 consecutive months. Common symptoms associated with menopause include hot flashes, sleep disturbances, mood changes, headaches, and heart palpitations. An article, co-authored by Dr. Manson, summarizes effective hormonal and non-hormonal treatments to manage menopausal symptoms [1].
The WHI’s longer-term follow-up of the treatment of these women, however, has demonstrated many nuanced findings [2]. For example, MHT’s risks and benefits are complex and vary based upon patient-level characteristics, including the age at which the therapy is initiated and the formulation of the MHT prescribed. Importantly, WHI was designed to assess the efficacy of MHT in preventing chronic disease, not to assess the efficacy or safety of MHT when used to treat menopausal symptoms. The average study participant was older, with over a decade since the start of their menopausal transition.
When considering any treatment, people should consult a health care professional, and MHT may be an option for some women, especially those who are experiencing menopausal symptoms and are at low risk for adverse events. The Food and Drug Administration (FDA) offers a fact sheet to answer questions and provide guidance about menopause and hormones, and has evaluated the risks and benefits of MHT for specific age groups of women [3].
In addition to WHI, there are two other valuable NIH-funded studies helping to make progress in our understanding of the health of midlife and older women:
• Study of Women’s Health Across the Nation (SWAN)
• Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH)
A major health concern for women during perimenopause, menopause, and post menopause is cardiovascular health. More research is needed to understand how different stages of menopause affect women’s cardiovascular health and how different doses and formulations of MHT may affect risk.
Among the many speakers at the Vivian W. Pinn Symposium will be Wendy Kohrt, a co-author on a recent comprehensive review of cardiovascular health and menopause [4]. She is director of the University of Colorado Specialized Centers of Research Excellence on Sex Differences (SCORE), Aurora. Also, a recent issue of ORWH’s Women’s Health in Focus at NIH discussed current NIH-funded research on menopause, resources, future menopause-related research, and more.
In response to a Congressional request to address NIH efforts related to women’s health research, ORWH hosted, along with the NIH Advisory Committee on Research on Women’s Health, “Advancing NIH Research on the Health of Women: A 2021 Conference.” The importance of menopause research as it relates to chronic debilitating conditions, which pose a significant burden on the health of women, was addressed during the conference, and the full report is available on the ORWH website.
Further, ORWH and partnering institutes released two notices of funding opportunities titled Understanding Chronic Conditions Understudied Among Women (R01 and R21), and ORWH sponsored the forthcoming Framework for the Consideration of Chronic Debilitating Conditions in Women from the National Academies of Sciences, Engineering, and Medicine.
I wish everyone a happy and healthy NWHW and look forward to gathering virtually for the 7th Annual Vivian W. Pinn Symposium. For more information and resources on menopause, visit the FDA’s Office of Women’s Health and NIH’s National Institute on Aging (NIA) websites. Also, My Menoplan, developed by NIA-funded researchers, offers information and personalized tools to help plan for perimenopause and menopause. Please stay connected to ORWH by visiting our website for updates; signing up for our monthly newsletter, The Pulse; liking us on Facebook; and following ORWH on Twitter.
References:
[1] Management of menopausal symptoms: A review. Crandall CJ, et al. JAMA. 2023 February 7: 329(5):405-420.
[2] Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. Manson JE, et al. JAMA. 2013 October 2: 310(13)1353-1368.
[3] Randomized trial evaluation of the benefits and risks of menopausal hormone therapy among women 50-59 years of age. Prentice RL, et al. Am J Epidemiol. 2021 February 1: 190(3):365-375.
[4] Body composition and cardiometabolic health across the menopause transition. Marlatt KL, et al. Obesity. 2022 January; 30(1)14-27.
Links:
Office of Research on Women’s Health (NIH)
7th Annual Vivian W. Pinn Symposium (ORWH)
Specialized Centers of Research Excellence on Sex Differences (SCORE) (ORWH)
National Women’s Health Week (Office on Women’s Health, U.S. Department of Health and Human Services, Rockville, MD)
Women’s Health Initiative (WHI)
Study of Women’s Health Across the Nation (SWAN)
Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) (Fred Hutchinson Cancer Center, Seattle)
Office of Women’s Health (U.S. Food and Drug Administration, Silver Spring, MD)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 30th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
10 Years of Protecting Public Health Through Tobacco Regulatory Research
Posted on by David M. Murray, Ph.D., NIH Office of Disease Prevention

“Kids are flocking to flavored, disposable e-cigarettes, study finds” – The Washington Post
“New ‘candy’ e-cigs catch fire after U.S. regulators stamp out Juul’s flavors” – Reuters
Headlines like these highlight a real challenge for people who want to protect kids from the harms of using tobacco products. While flavors, such as mint, menthol, watermelon, and apple pie are safe to consume in food products, inhaling them in tobacco products can be harmful and put the health of our kids at risk.+
A special kind of research is needed to help public health authorities keep up with the latest changes and trends in tobacco products. That includes studying how these flavored tobacco products are attractively marketed to children and how quickly many started using them.
In 2013, NIH and the Food and Drug Administration (FDA) launched a unique interagency partnership called the Tobacco Regulatory Science Program (TRSP), directed by Helen Meissner. It aims to reduce the public health impact of tobacco product use across the country. The NIH administers the research program through the Office of Disease Prevention (ODP), which I lead, to help inform FDA’s tobacco regulatory priorities.
This unique partnership also represents a new field of study called tobacco regulatory research. It informs proposed regulations for tobacco products based on strong scientific evidence. The TRSP brings together scientists from diverse fields, such as epidemiology, chemistry, toxicology, addiction, and psychology, to shed light on why people try and continue to use tobacco, how tobacco use affects health, and which policies might help reduce the risk of harm.
Now celebrating its 10th anniversary, this extremely productive partnership has resulted in more than 400 research grants, all peer-reviewed and designed to increase our understanding of existing and emerging tobacco products and their associated health risks.
Our research includes studies showing that menthol in cigarettes makes it easier to start smoking by reducing the harshness of tobacco [1]. People who smoke menthol cigarettes also show more signs of nicotine dependence and, therefore, are less likely to successfully quit. The research shows this is because menthol interacts with nicotine in the brain, making nicotine even more addictive.
Additionally, researchers have explored how marketing and promotion of menthol and flavored tobacco products have targeted Black and LGBTQ+ people, socioeconomically disadvantaged populations, and people with mental health challenges. These studies show that this direct marketing has contributed to the burden of tobacco-related disease among these groups and widened health inequities [2].
The TRSP also has a real-world impact on shaping tobacco policy. In April 2022, the program’s sponsored research was cited in FDA-proposed rules to prohibit menthol as a characterizing flavor in cigarettes and ban all characterizing flavors (other than tobacco) in cigars [3]. These tobacco product standards will have a huge impact on public health by reducing youth experimentation with products like cigarettes, cigars, and cigarillos and increasing the number of people who quit smoking.
Many jurisdictions have already banned flavored tobacco products. Through our partnership with the FDA, TRSP-funded researchers have started evaluating the impact of these policies on tobacco use and public health. The need for research continues as we seek to understand how new tobacco products affect people’s use, attitudes, and health.
However, tobacco products that have the potential to addict a new generation to nicotine continue to be marketed. For example, new products that use “ice-hybrid” flavors which combine cooling and fruity/sweet properties, such as raspberry ice, are being used more often than either fruity/sweet or menthol/mint among young adult e-cigarette users [4]. Illegally marketed, but novel, flavored oral nicotine products, such as gummies and pouches, also are gaining appeal among young people. The dynamic nature of the tobacco market emphasizes the importance of TRSP to support research on tobacco products, directly informing tobacco regulation.
The success of TRSP over the past 10 years demonstrates how establishing a research pipeline that directly informs regulation can lead to effective, evidence-based health policies. The high output of research on the effects of new and emerging tobacco products, such as the appeal and addictiveness of flavored e-cigarettes, provides FDA with data to inform regulatory actions. This partnership is truly helping regulators and policymakers turn scientific discovery into actions designed to protect public health.
References:
[1] Use of menthol cigarettes, smoking frequency, and nicotine dependence among US youth. Leas EC, Benmarhnia T, Strong DR, Pierce JP. JAMA Netw Open. 2022 Jun 1;5(6):e2217144.
[2] Menthol smoking and related health disparities. Centers for Disease Control and Prevention, June 27, 2022.
[3] FDA proposes rules prohibiting menthol cigarettes and flavored cigars to prevent youth initiation, significantly reduce tobacco-related disease and death. FDA News Release, April 28, 2022.
[4] ‘Ice’ flavoured e-cigarette use among young adults. Leventhal A, Dai H, Barrington-Trimis J, Sussman S. Tob Control. 2023 Jan;32(1):114-117.
Links:
Smokefree.gov (U.S. Department of Health and Human Services, Washington, D.C.)
Office of Disease Prevention (NIH)
Tobacco Regulatory Science Program (ODP)
Director’s Messages (ODP)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 29th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
NIH HEAL Initiative Meets People Where They Are
Posted on by Rebecca Baker, Ph.D., NIH Helping to End Addiction Long-term® (HEAL) Initiative

The opioid crisis continues to devastate communities across America. Dangerous synthetic opioids, like fentanyl, have flooded the illicit drug supply with terrible consequences. Tragically, based on our most-recent data, about 108,000 people in the U.S. die per year from overdoses of opioids or stimulants [1]. Although this complex public health challenge started from our inability to treat pain effectively, chronic pain remains a life-altering problem for 50 million Americans.
To match the size and complexity of the crisis, in 2018 NIH developed the NIH Helping to End Addiction Long-term® (HEAL) Initiative, an aggressive effort involving nearly all of its 27 institutes and centers. Through more than 1,000 research projects, including basic science, clinical testing of new and repurposed drugs, research with communities, and health equity research, HEAL is dedicated to building a new future built on hope.
In this future:
- A predictive tool used during a health visit personalizes treatment for back pain. The tool estimates the probability that a person will benefit from physical therapy, psychotherapy, or surgery.
- Visits to community health clinics and emergency departments serve as routine opportunities to prevent and treat opioid addiction.
- Qualified school staff and pediatricians screen all children for behavioral and other mental health conditions that increase risk for harmful developmental outcomes, including opioid misuse.
- Infants born exposed to opioids during a mother’s pregnancy receive high-quality care—setting them up for a healthy future.
Five years after getting started (and interrupted by a global pandemic), HEAL research is making progress toward achieving this vision. I’ll highlight three ways in which scientific solutions are meeting people where they are today.
A Window of Opportunity for Treatment in the Justice System
Sadly, jails and prisons are “ground zero” for the nation’s opioid crisis. Eighty-five percent of people who are incarcerated have a substance use disorder or a history of substance use. Our vision at HEAL is that every person in jail, prison, or a court-supervised program receives medical care, which includes effective opioid use disorder treatment.
Some research results already are in supporting this approach: A recent HEAL study learned that individuals who had received addiction treatment while in one Massachusetts jail were about 30 percent less likely to be arrested, arraigned, or incarcerated again compared with those incarcerated during the same time period in a neighboring jail that did not offer treatment [2]. Research from the HEAL-supported Justice Community Opioid Innovation Network also is exploring public perceptions about opioid addiction. One such survey showed that most U.S. adults see opioid use disorder as a treatable medical condition rather than as a criminal matter [3]. That’s hopeful news for the future.
A Personalized Treatment Plan for Chronic Back Pain
Half of American adults live with chronic back pain, a major contributor to opioid use. The HEAL-supported Back Pain Consortium (BACPAC) is creating a whole-system model for comprehensive testing of everything that contributes to chronic low back pain, from anxiety to tissue damage. It also includes comprehensive testing of promising pain-management approaches, including psychotherapy, antidepressants, or surgery.
Refining this whole-system model, which is nearing completion, includes finding computer-friendly ways to describe the relationship between the different elements of pain and treatment. That might include developing mathematical equations that describe the physical movements and connections of the vertebrae, discs, and tendons.
Or it might include an artificial intelligence technique called machine learning, in which a computer looks for patterns in existing data, such as electronic health records or medical images. In keeping with HEAL’s all-hands-on-deck approach, BACPAC also conducts clinical trials to test new (or repurposed) treatments and develop new technologies focused on back pain, like a “wearable muscle” to help support the back.
Harnessing Innovation from the Private Sector
The HEAL research portfolio spans basic science to health services research. That allows us to put many shots on goal that will need to be commercialized to help people. Through its research support of small businesses, HEAL funding offers a make-or-break opportunity to advance a great idea to the marketplace, providing a bridge to venture capital or other larger funding sources needed for commercialization.
This bridge also allows HEAL to invest directly in the heart of innovation. Currently, HEAL funds nearly 100 such companies across 20 states. While this is a relatively small portion of all HEAL research, it is science that will make a difference in our communities, and these researchers are passionate about what they do to build a better future.
A couple of current examples of this research passion include: delivery of controlled amounts of non-opioid pain medications after surgery using a naturally absorbable film or a bone glue; immersive virtual reality to help people with opioid use disorder visualize the consequences of certain personal choices; and mobile apps that support recovery, taking medications, or sensing an overdose.
In 2023, HEAL is making headway toward its mission to accelerate development of safe, non-addictive, and effective strategies to prevent and treat pain, opioid misuse, and overdose. We have 314 clinical trials underway and 41 submissions to the Food and Drug Administration to begin clinical testing of investigational new drugs or devices: That number has doubled in the last year. More than 100 projects alone are addressing back pain, and more than 200 projects are studying medications for opioid use disorder.
The nation’s opioid crisis is profoundly difficult and multifaceted—and it won’t be solved with any single approach. Our research is laser-focused on its vision of ending addiction long-term, including improving pain management and expanding access to underused, but highly effective, addiction medications. Every day, we imagine a better future for people with physical and emotional pain and communities that are hurting. Hundreds of researchers and community members across the country are working to achieve a future where people and communities have the tools they need to thrive.
References:
[1] Provisional drug overdose death counts. Ahmad FB, Cisewski JA, Rossen LM, Sutton P. National Center for Health Statistics. 2023.
[2] Recidivism and mortality after in-jail buprenorphine treatment for opioid use disorder. Evans EA, Wilson D, Friedmann PD. Drug Alcohol Depend. 2022 Feb 1;231:109254.
[3] Social stigma toward persons with opioid use disorder: Results from a nationally representative survey of U.S. adults. Taylor BG, Lamuda PA, Flanagan E, Watts E, Pollack H, Schneider J. Subst Use Misuse. 2021;56(12):1752-1764.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
NIH Helping to End Addiction Long-term® (HEAL) Initiative
Video: The NIH HEAL Initiative–HEAL Is Hope
Justice Community Opioid Innovation Network (HEAL)
Back Pain Consortium Research Program (HEAL)
NIH HEAL Initiative 2023 Annual Report (HEAL)
Small Business Programs (HEAL)
Rebecca Baker (HEAL)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 28th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
New Tool Predicts Response to Immunotherapy in Lung Cancer Patients
Posted on by Douglas M. Sheeley, Sc.D., NIH Common Fund

With just a blood sample from a patient, a promising technology has the potential to accurately diagnose non-small cell lung cancer (NSCLC), the most-common form of the disease, more than 90 percent of the time. The same technology can even predict from the same blood sample whether a patient will respond well to a targeted immunotherapy treatment.
This work is a good example of research supported by the NIH Common Fund. Many Common Fund programs support development of new tools that catalyze research across the full spectrum of biomedical science without focusing on a single disease or organ system.
The emerging NSCLC prediction technology was developed as part of our Extracellular RNA Communication Program. The program develops technologies to understand RNA circulating in the body, known as extracellular RNA (exRNA). These molecules can be easily accessed in bodily fluids such as blood, urine, and saliva, and they have enormous potential as biomarkers to better understand cancer and other diseases.
When the body’s immune system detects a developing tumor, it activates various immune cells that work together to kill the suspicious cells. But many tumors have found a way to evade the immune system by producing a protein called PD-L1.
Displayed on the surface of a cancer cell, PD-L1 can bind to a protein found on immune cells with the similar designation of PD-1. The binding of the two proteins keeps immune cells from killing tumor cells. One type of immunotherapy interferes with this binding process and can restore the natural ability of the immune system to kill the tumor cells.
However, tumors differ from person to person, and this form of cancer immunotherapy doesn’t work for everyone. People with higher levels of PD-L1 in their tumors generally have better response rates to immunotherapy, and that’s why oncologists test for the protein before attempting the treatment.
Because cancer cells within a tumor can vary greatly, a single biopsy taken at a single site in the tumor may miss cells with PD-L1. In fact, current prediction technologies using tissue biopsies correctly predict just 20 – 40 percent of NSCLC patients who will respond well to immunotherapy. This means some people receive immunotherapy who shouldn’t, while others don’t get it who might benefit.
To improve these predictions, a research team led by Eduardo Reátegui, The Ohio State University, Columbus, engineered a new technology to measure exRNA and proteins found within and on the surface of extracellular vesicles (EVs) [1]. EVs are tiny molecular containers released by cells. They carry RNA and proteins (including PD-L1) throughout the body and are known to play a role in communication between cells.
As the illustration above shows, EVs can be shed from tumors and then circulate in the bloodstream. That means their characteristics and internal cargo, including exRNA, can provide insight into the features of a tumor. But collecting EVs, breaking them open, and pooling their contents for assessment means that molecules occurring in small quantities (like PD-L1) can get lost in the mix. It also exposes delicate exRNA molecules to potential breakdown outside the protective EV.
The new technology solves these problems. It sorts and isolates individual EVs and measures both PD-1 and PD-L1 proteins, as well as exRNA that contains their genetic codes. This provides a more comprehensive picture of PD-L1 production within the tumor compared to a single biopsy sample. But also, measuring surface proteins and the contents of individual EVs makes this technique exquisitely sensitive.
By measuring proteins and the exRNA cargo from individual EVs, Reátegui and team found that the technology correctly predicted whether a patient had NSCLC 93.2 percent of the time. It also predicted immunotherapy response with an accuracy of 72.2 percent, far exceeding the current gold standard method.
The researchers are working on scaling up the technology, which would increase precision and allow for more simultaneous measurements. They are also working with the James Comprehensive Cancer Center at The Ohio State University to expand their testing. That includes validating the technology using banked clinical samples of blood and other bodily fluids from large groups of cancer patients. With continued development, this new technology could improve NSCLC treatment while, critically, lowering its cost.
The real power of the technology, though, lies in its flexibility. Its components can be swapped out to recognize any number of marker molecules for other diseases and conditions. That includes other cancers, neurodegenerative diseases, traumatic brain injury, viral diseases, and cardiovascular diseases. This broad applicability is an example of how Common Fund investments catalyze advances across the research spectrum that will help many people now and in the future.
Reference:
[1] An immunogold single extracellular vesicular RNA and protein (AuSERP) biochip to predict responses to immunotherapy in non-small cell lung cancer patients. Nguyen LTH, Zhang J, Rima XY, Wang X, Kwak KJ, Okimoto T, Amann J, Yoon MJ, Shukuya T, Chiang CL, Walters N, Ma Y, Belcher D, Li H, Palmer AF, Carbone DP, Lee LJ, Reátegui E. J Extracell Vesicles. 11(9):e12258. doi: 10.1002/jev2.12258.
Links:
Video: Unlocking the Mysteries of Extracellular RNA Communication (Common Fund)
Extracellular RNA Communication Program (ERCC) (Common Fund)
Upcoming Meeting: ERCC19 Research Meeting (May 1-2, 2023)
Eduardo Reátegui Group for Bioengineering Research (The Ohio State University College of Engineering, Columbus)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 27th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
A Whole Person Approach to Lifting the Burden of Chronic Pain Among Service Members and Veterans
Posted on by Helene M. Langevin, M.D., National Center for Complementary and Integrative Health

Chronic pain and its companion crisis of opioid misuse have taken a terrible toll on Americans. But the impact has been even greater on U.S. service members and veterans, who often deal with the compounded factors of service-related injuries and traumatic stress.
For example, among soldiers in a leading U.S. Army unit, 44 percent had chronic pain and 15 percent used opioids after a combat deployment. That compares to 26 percent and 4 percent, respectively, in the general population [1,2].
This disproportionate burden of chronic pain among veterans [3] and service members led NIH’s National Center for Complementary and Integrative Health (NCCIH) to act. We forged a collaboration in 2017 across NIH, U.S. Department of Defense (DOD), and U.S. Department of Veteran’s Affairs (VA) to establish the Pain Management Collaboratory (PMC).
The PMC’s research focusing on the implementation and evaluation of nondrug approaches for the management of pain is urgently needed in the military and across our entire country. Nondrug approaches require a shift in thinking. Rather than focusing solely on blocking pain temporarily using analgesics, nondrug approaches work with the mind and body to promote the resolution of chronic pain and the long-term restoration of health through techniques and practices such as manual therapy, yoga, and mindfulness-based interventions.
Addressing chronic pain in ways that don’t only rely on drugs means addressing underlying issues, such as joints and connective tissue that lack adequate movement or training our brains to “turn down the volume” on pain signals. Using mind and body practices to reduce pain can help promote health in other ways. Possible “fringe benefits” include better sleep, more energy for physical activity, a better mindset for making good nutritional choices, and/or improved mood.
Indeed, there is a growing body of research on the benefits of nondrug approaches to address chronic pain. What is so powerful about PMC is it puts this knowledge to work by embedding research within military health care settings.
The PMC supports a shared resource center and 11 large-scale pragmatic clinical trials. Within this real-world health care setting, the clinical trials have enrolled more than 8,200 participants across 42 veteran and military health systems. These studies offer both strength in numbers and insights into what happens when learnings from controlled clinical trials collide with the realities of health care delivery and the complexities of daily life. [4]
Central to the PMC partnership is whole person health. Too often, we see health through the prism of separate parts—for example, a person’s cardiovascular, digestive, and mental health problems are viewed as co-occurring rather than as interrelated conditions. A whole person framework—a central focus of NCCIH’s current Strategic Plan—brings the parts back together and recognizes that health exists across multiple interconnected body systems and domains: biological, behavioral, social, and environmental.
The VA’s implementation of a whole health model [5] and their unique closed-loop health care system offers an opportunity to deliver care, conduct research, and illustrate what happens when people receive coordinated care that treats the whole person. In fact, VA’s leadership in this area was the impetus for a recent report by the National Academies of Sciences, Engineering, and Medicine. The report underscored the importance of implementing whole person health care in all settings and for every American.
There are many opportunities ahead for this interagency collaboration. It will help to achieve an important shift, from treating problems one at a time to promoting overall military readiness, resilience, and well-being for U.S. service members and veterans.
Congress appropriated $5 million to NCCIH in fiscal year 2023 to enhance pain research with a special emphasis on military populations. These additional resources will allow NCCIH to support more complex studies in understanding how multiple therapeutic approaches that impact multiple body systems can impact chronic pain.
Meanwhile, programs like the DOD’s Consortium for Health and Military Performance (CHAMP) will continue to translate these lessons learned into accessible pain management information that service members can use in promoting and maintaining their health.
While the PMC’s research program specifically targets the military community, this growing body of knowledge will benefit us all. Understanding how to better manage chronic pain and offering more treatment options for those who want to avoid the risks of opioids will help us all build resilience and restore health of the whole person.
References:
[1] Chronic pain and opioid use in US soldiers after combat deployment. Toblin RL, Quartana PJ, Riviere LA, Walper KC, Hoge CW. JAMA Intern. Med. 2014 Aug;174(8):1400-1401.
[2] Pain and opioids in the military: We must do better. Jonas WB, Schoomaker EB. JAMA Intern. Med. 2014 Aug;174(8):1402-1403
[3] Severe pain in veterans: The effect of age and sex, and comparisons with the general population. Nahin RL. J Pain. 2017 Mar; 18(3):247-254.
[4] Justice and equity in pragmatic clinical trials: Considerations for pain research within integrated health systems. Ali J, Davis AF, Burgess DJ, Rhon DI, Vining R, Young-McCaughan S, Green S, Kerns RD. Learn Health Sys. 2021 Oct 19;6(2): e10291
[5] The APPROACH trial: Assessing pain, patient-reported outcomes, and complementary and integrative health. Zeliadt S, Coggeshall S, Thomas E, Gelman H, Taylor S. Clin. Trials. 2020 Aug;17(4):351-359.
Links:
National Center for Complementary and Integrative Health (NIH)
NCCIH Strategic Plan FY 2021-2025: Mapping a Pathway to Research on Whole Person Health (NIH)
Pain Management Collaboratory (Yale University, New Haven, CT)
Whole Health (U.S Department of Veteran’s Affairs, Washington, D.C.)
Consortium for Health and Military Performance (Department of Defense, Bethesda, MD)
Achieving Whole Health: A New Approach for Veterans and the Nation. (National Academies of Sciences, Engineering, and Medicine, Washington, D.C.)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 26th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
RECOVER: What Clinical Research Comes Next for Helping People with Long COVID

“I connected with RECOVER to be a part of the answers that I was looking for when I was at my worst.” Long COVID patient and RECOVER representative, Nitza Rochez (Bronx, NY)
People, like Nitza Rochez, who are living with Long COVID—the wide-ranging health issues that can follow an infection with SARS-CoV-2, the coronavirus that causes COVID-19—experience disabling symptoms with significant physical, emotional and financial consequences.
The NIH has been engaging and listening to Nitza and others living with Long COVID even before the start of its Researching COVID to Enhance Recovery (RECOVER) Initiative. But now, with the launch of RECOVER, patients and those with affected family or community members have joined researchers, clinicians, and experts in their efforts to unlock the mysteries of Long COVID. All have come together to understand what causes the condition, identify who is most at risk, and determine how to prevent and treat it.
RECOVER is unprecedented in its size and scope as the most-diverse, deeply characterized cohort of Long COVID patients. We’ve enlisted the help of many patient volunteers, who have enrolled in observational studies designed to help researchers learn as much as possible about people who have Long COVID.
Indeed, thousands of research participants are now providing health information and undergoing in-depth medical evaluations and tests, enabling investigators to look for trends. Additionally, studies of millions of electronic medical records are providing insights about those who have received care during the pandemic. More than 40 studies are being conducted to identify the causes of disease, potential biomarkers of Long COVID, and new therapeutic targets.
In all, RECOVER’s research assets are voluminous. They involve invaluable contributions from many people and communities, including research volunteers, research investigators, and clinical specialists. In addition, millions of health records and numerous related tissues and specimens are being analyzed for possible leads.
At the center of it all is the National Community Engagement Group (NCEG). The NCEG is comprised of people living with Long COVID and those representing others living with the condition, and it is truly instrumental to the initiative’s progress in understanding how and why SARS-CoV-2 impacts people in different ways. It’s also helping researchers learn why some people recover while others do not.
So far, we’ve learned that people hospitalized with COVID-19 are twice as likely to have Long COVID than those who were not hospitalized for infection. We’ve also learned that members of racial and ethnic minority groups with Long COVID were more likely to have been hospitalized with COVID-19.
Similarly, disparities in Long COVID exist within those living in areas with particular environmental exposures [1], and those who were already burdened by other diseases and conditions—such as diabetes and chronic pulmonary disease [2]. We’ve also discovered that the certain types of symptoms of Long COVID are consistent among patients regardless of which SARS-CoV-2 variant caused their initial infection. Yet, people infected with the earlier variants have a higher number of symptoms than those infected with more recent variants.
Patient experiences have guided and will continue to guide the study designs and trajectory of RECOVER. Now, fueled by the knowledge that we have gained, RECOVER is preparing to advance to the next phase of discovery—testing interventions in clinical trials to see if they can help people with Long COVID.
To prepare, we are beginning to identify potential clinical trial sites. This important step will help us to find the right places with the right staff and capabilities for enrolling the appropriate patient populations needed to implement the studies. We’ll ensure that the public knows when these upcoming clinical trials are ready to enroll.
Of course, the design of these RECOVER clinical trials will be critical, and insights gained from patients have been key in this process. Results from RECOVER study questionnaires, surveys, and discussions with people experiencing Long COVID identified symptom clusters considered to be the most significant and burdensome to patients. These include sleep disorders, “brain fog” (trouble thinking clearly), exercise intolerance and fatigue, and nervous system dysfunction affecting people’s ability to regulate normal body functions like heart rate and body temperature.
These patient observations have effectively guided the design of the clinical trials that will evaluate whether certain interventions and therapies can help alleviate symptoms that are part of these specific clusters. We’re excited to be advancing toward this phase of the initiative and, again, are very grateful to patient representatives like Nitza, quoted above, for getting us to this phase.
Effective evaluation of those treatments will be important, too. Early in the pandemic, while many clinical trials were launching, most were not large enough or did not have the appropriate objectives to define effective treatments for acute COVID-19. This left clinicians with few clear options when faced with patients needing help.
Learning from this experience, the RECOVER trials will be harmonized to ensure coordinated and efficient evaluation of interventions—in other words, all potential therapies will be using the same protocols platforms and the same data elements. This consistency accelerates our understanding and strengthens the certainty of findings.
Given the widespread and diverse impact that the virus has on the body, it is highly likely that more than one treatment will be needed for each kind of patient experience. Finding solutions for everyone—people of all races, ethnicities, genders, ages, and geographic locations—is paramount.
RECOVER patient representative, Juan Lewis, of San Antonio shared with us, “In April 2020, I was fighting for my life, and today I fight for my quality of life. COVID impacted me physically, mentally, socially, and financially.”
For people like Juan who are experiencing debilitating Long COVID symptoms, we know that finding answers as quickly as possible is critical. As we look ahead to the next 12 months, we’ll continue the studies evaluating the underlying causes, risk factors, and outcomes of Long Covid, and we anticipate significant scientific progress on research leading to Long COVID treatments.
Keep an eye on the RECOVER website for updates on our progress, and published findings.
References:
[1] Identifying environmental risk factors for post-acute sequelae of SARS-CoV-2 infection: An EHR-based cohort study from the recover program. Zhang Y, Hu H, Fokaidis V, V CL, Xu J, Zang C, Xu Z, Wang F, Koropsak M, Bian J, Hall J, Rothman RL, Shenkman EA, Wei WQ, Weiner MG, Carton TW, Kaushal R. Environ Adv. 2023 Apr;11:100352.
[2] Identifying who has long COVID in the USA: a machine learning approach using N3C data. Pfaff ER, Girvin AT, Bennett TD, Bhatia A, Brooks IM, Deer RR, Dekermanjian JP, Jolley SE, Kahn MG, Kostka K, McMurry JA, Moffitt R, Walden A, Chute CG, Haendel MA; N3C Consortium. Lancet Digit Health. 2022 Jul;4(7):e532-e541.
Links:
RECOVER: Researching COVID to Enhance Recovery
Long COVID: Ask NIH Leader about Latest Research (YouTube)
NIH Builds Large Nationwide Study Population of Tens of Thousands to Support Research on Long-Term Effects of COVID-19, NIH News Release, September 15, 2021
Understanding Long-Term COVID-19 Symptoms and Enhancing Recovery, NIH Director’s Blog, October 4, 2022.
NIH RECOVER Research Identifies Potential Long COVID Disparities. NIH News Release, February 16, 2023.
NIH RECOVER Listening Session, June 2021 (NIH Videocast)
NIH RECOVER Listening Session: Understanding Long COVID Across Communities of Color and Those Hardest Hit by COVID, January 21, 2022 (NIH Videocast)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 25th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
All of Us Research Program Participants Fuel Both Scientific and Personal Discovery
Posted on by Josh Denny, M.D., M.S., All of Us Research Program
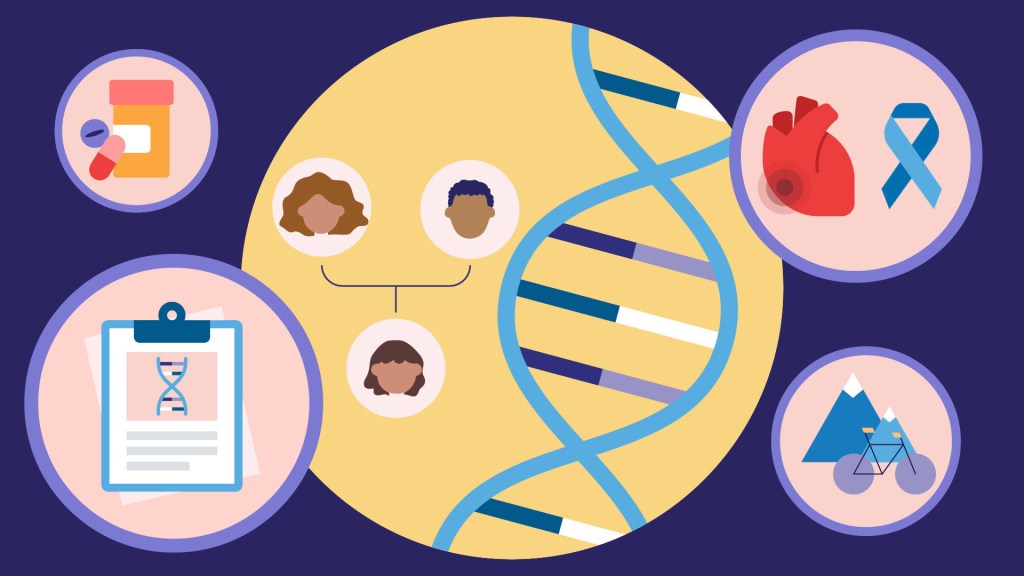
The NIH’s All of Us Research Program is a historic effort to create an unprecedented research resource that will speed biomedical breakthroughs, transform medicine and advance health equity. To create this resource, we are enrolling at least 1 million people who reflect the diversity of the United States.
At the program’s outset, we promised to make research a two-way street by returning health information to our participant partners. We are now delivering on that promise. We are returning personalized health-related DNA reports to participants who choose to receive them.
That includes me. I signed up to receive my “Medicine and Your DNA” and “Hereditary Disease Risk” reports along with nearly 200,000 other participant partners. I recently read my results, and they hit home, revealing an eye-opening connection between my personal and professional lives.
First, the professional. Before coming to All of Us, I was a practicing physician and researcher at Vanderbilt University, Nashville, TN, where I studied how a person’s genes might affect his or her response to medications. One of the drug-gene interactions that I found most interesting is related to clopidogrel, a drug commonly prescribed to keep arteries open after a major cardiovascular event, like a heart attack, stroke, or placement of a stent.
People with certain gene variations are not able to process this medication well, leaving them in a potentially risky situation. The patient and their health care provider may think the condition is being managed. But, since they can’t process the medication, the patient’s symptoms and risks are likely to increase.
The impact on patients has been seen in numerous studies, including one that I published with colleagues last year in the Journal of Stroke and Cerebrovascular Disease [1]. We found that stroke risk is three times higher in patients who were poor responders to clopidogrel and treated with the drug following a “mini-stroke”—also known as a transient ischemic attack. Other studies have shown that major cardiovascular events were 50 percent more common in individuals who were poor responders to clopidogrel [2]. Importantly, there are alternative therapies that work well for people with this genetic variant.
Now, the personal. Reading my health-related results, I learned that I carry some of these very same gene variations. So, if I ever needed a medicine to manage my risk of blood clots, clopidogrel would not likely work well for me.
Instead, should I ever need treatment, my provider and I could bypass this common first-line therapy and choose an alternate medicine. Getting the right treatment on the first try could cut my chances of a heart attack in half. The benefits of this knowledge don’t stop with me. By choosing to share my findings with family members who may have inherited the same genetic variations, they can discuss it with their health care teams.
Other program participants who choose to receive results will experience the same process of learning more about their health. Nearly all will get actionable information about how their body may process certain medications. A small percentage, 2 to 3 percent, may learn they’re at higher risk of developing several severe hereditary health conditions, such as certain preventable heart diseases and cancers. The program will provide a genetic counselor at no cost to all participants to discuss their results.
To enroll participants who reflect the country’s diverse population, All of Us partners with trusted community organizations around the country. Inclusion is vitally important in the field of genomics research, where available data have long originated mostly from people of European ancestry. In contrast, about 50 percent of the All of Us’ genomic data come from individuals who self-identify with a racial or ethnic minority group.
More than 3,600 research projects are already underway using data contributed by participants from diverse backgrounds. What’s especially exciting about this “ecosystem” of discovery between participants and researchers is that, by contributing their data, participants are helping researchers decode what our DNA is telling us about health across all types of conditions. In turn, those discoveries will deepen what participants can learn.
Those who have stepped up to join All of Us are the heartbeat of this historic research effort to advance personalized approaches in medicine. Their contributions are already fueling new discoveries in numerous areas of health.
At the same time, making good on our promises to our participant partners ensures that the knowledge gained doesn’t only accumulate in a database but is delivered back to participants to help advance their own health journeys. If you’re interested in joining All of Us, we welcome you to learn more.
References:
[1] CYP2C19 loss-of-function is associated with increased risk of ischemic stroke after transient ischemic attack in intracranial atherosclerotic disease. Patel PD, Vimalathas P, Niu X, Shannon CN, Denny JC, Peterson JF, Chitale RV, Fusco MR. J Stroke Cerebrovasc Dis. 2021 Feb;30(2):105464.
[2] Predicting clopidogrel response using DNA samples linked to an electronic health record. Delaney JT, Ramirez AH, Bowton E, Pulley JM, Basford MA, Schildcrout JS, Shi Y, Zink R, Oetjens M, Xu H, Cleator JH, Jahangir E, Ritchie MD, Masys DR, Roden DM, Crawford DC, Denny JC. Clin Pharmacol Ther. 2012 Feb;91(2):257-263.
Links:
Join All of Us (All of Us/NIH)
NIH’s All of Us Research Program returns genetic health-related results to participants, NIH News Release, December 13, 2022.
NIH’s All of Us Research Program Releases First Genomic Dataset of Nearly 100,000 Whole Genome Sequences, NIH News Release, March 17, 2022.
Funding and Program Partners (All of Us)
Medicine and Your DNA (All of Us)
Clopidogrel Response (National Library of Medicine/NIH)
Hereditary Disease Risk (All of Us)
Preparing for DNA Results: What Is a Genetic Counselor? (All of Us)
Research Projects Directory (All of Us)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 24th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Celebrating the Power of Connection This Holiday Season
Posted on by Lawrence Tabak, D.D.S., Ph.D.
Happy holidays to one and all! This short science video brings to mind all those twinkling lights now brightening the night, as we mark the beginning of winter and shortest day of the year. This video also helps to remind us about the power of connection this holiday season.
It shows a motor neuron in a mouse’s primary motor cortex. In this portion of the brain, which controls voluntary movement, heavily branched neural projections interconnect, sending and receiving signals to and from distant parts of the body. A single motor neuron can receive thousands of inputs at a time from other branching sensory cells, depicted in the video as an array of blinking lights. It’s only through these connections—through open communication and cooperation—that voluntary movements are possible to navigate and enjoy our world in all its wonder. One neuron, like one person, can’t do it all alone.
This power of connection, captured in this award-winning video from the 2022 Show Us Your Brains Photo and Video contest, comes from Forrest Collman, Allen Institute for Brain Science, Seattle. The contest is part of NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative.
In the version above, we’ve taken some liberties with the original video to enhance the twinkling lights from the synaptic connections. But creating the original was quite a task. Collman sifted through reams of data from high-resolution electron microscopy imaging of the motor cortex to masterfully reconstruct this individual motor neuron and its connections.
Those data came from The Machine Intelligence from Cortical Networks (MICrONS) program, supported by the Intelligence Advanced Research Projects Activity (IARPA). It’s part of the Office of the Director of National Intelligence, one of NIH’s governmental collaborators in the BRAIN Initiative.
The MICrONS program aims to better understand the brain’s internal wiring. With this increased knowledge, researchers will develop more sophisticated machine learning algorithms for artificial intelligence applications, which will in turn advance fundamental basic science discoveries and the practice of life-saving medicine. For instance, these applications may help in the future to detect and evaluate a broad range of neural conditions, including those that affect the primary motor cortex.
Pretty cool stuff. So, as you spend this holiday season with friends and family, let this video and its twinkling lights remind you that there’s much more to the season than eating, drinking, and watching football games.
The holidays are very much about the power of connection for people of all faiths, beliefs, and traditions. It’s about taking time out from the everyday to join together to share memories of days gone by as we build new memories and stronger bonds of cooperation for the years to come. With this in mind, happy holidays to one and all.
Links:
“NIH BRAIN Initiative Unveils Detailed Atlas of the Mammalian Primary Motor Cortex,” NIH News Release, October 6, 2021
Forrest Collman (Allen Institute for Brain Science, Seattle)
Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your Brains Photo and Video Contest (BRAIN Initiative)
Next Page

