public health
Visiting West Virginia University and Sharing Vision for Enhancing the Nation’s Health
Posted on by Dr. Monica M. Bertagnolli

NIH Director Monica Bertagnolli with Senator Shelley Moore Capito (left), and Dr. Ming Lei, Vice Dean of Research at the WVU School of Medicine (right), during a visit to West Virginia University Health Sciences Campus on March 27, 2024. Credit: (all photos) The Office of U.S. Senator Shelley Moore Capito (R-W.Va.).
In March, I had the pleasure of joining Senator Shelley Moore Capito in my first ever visit to West Virginia University Health Sciences Campus in Morgantown, where I got to witness NIH-supported research in action. On our tour, we visited a number of labs and scientific centers, learning about work being done in important areas of research including stroke, cancer, sleep trials, vaccine development, diabetes, cardiovascular heath, and more. I also gave a presentation in which I shared my vision, discussing my guiding principles and priorities as NIH Director, and I spoke about some of the biggest challenges in American health care and what NIH might do to address them. We were joined by Senator Joe Manchin and many distinguished WVU leaders and researchers, and I’m so grateful for the time and warm welcome they provided. To see such wide-ranging and vital research being done was inspiring, and I look forward to similar visits in the future.
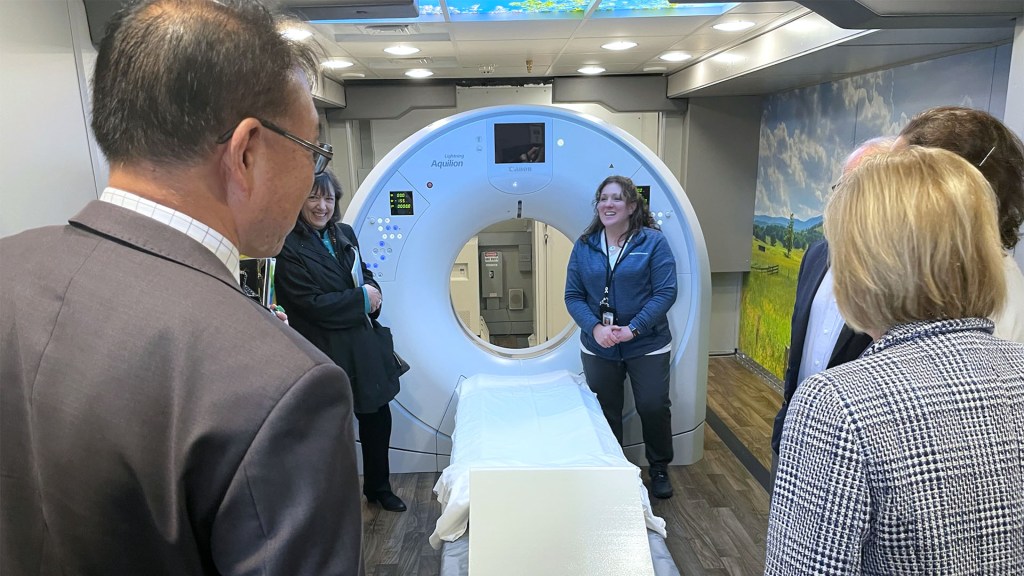
The tour included a visit to WVU Cancer Institute’s mobile lung cancer screening unit, LUCAS, which travels the state, bringing lung cancer screening to people in rural areas.

Got My Flu Shot
Posted on by Lawrence Tabak, D.D.S., Ph.D.

NIH Collaboration Seeks to Help Understand U.S. Burden of Health Disparities: Why Your County Matters
Posted on by Eliseo J. Pérez-Stable, M.D., National Institute on Minority Health and Health Disparities

Since the early 1990s, federal support of research has increased to understand minority health and identify and address health disparities. Research in these areas has evolved from a starting point of developing a basic descriptive understanding of health disparities and who is most affected. Now, it is discovering the underlying complexity of factors involved in health outcomes to inform interventions and reduce these disparities.
One of these many factors is where we live, learn, work, and play and how that affects different people. A group of NIH scientists and their colleagues recently published a study in the journal The Lancet that they hope is a step toward better understanding geographic disparities and their role in health equity [1].

As Director of NIH’s National Institute on Minority Health and Health Disparities (NIMHD), I worked with NIMHD’s Scientific Director, Anna María Nápoles, to conceive the study and establish the Global Burden of Disease (GBD) U.S. Health Disparities Collaborators at NIH with five NIH Institutes and two Offices. Through this collaboration, NIH funded the Institute for Health Metrics and Evaluation (IHME), University of Washington to conduct the analysis. The IHME has worked for 30 years on the GBD project in over 200 countries.
The Lancet paper offered the first comprehensive U.S. county-level life expectancy estimates to highlight the significant gaps that persist among racial and ethnic populations across the nation. The analysis revealed that despite overall life expectancy gains of 2.3 years from 2000–2019, Black populations experienced shorter life expectancy than White populations.
In addition, American Indian and Alaska Native populations’ life expectancy did not improve and, in fact, decreased in most counties. We found national-level life expectancy advantages for Hispanic/Latino and Asian populations ranging from three to seven years, respectively, compared to White populations. But there were notable exceptions for Hispanic/Latino populations in selected counties in the Southwest.
Certainly the most-alarming trend identified in the paper was that during the study’s last 10 years (2010–2019), life expectancy growth was stagnant across all races and ethnicities. Moreover, 60 percent of U.S. counties experienced a decrease in life expectancy.
While these findings provide an important frame for how disparities exist along many dimensions—by race, ethnicity, and geographic region—they also highlight these differences within our local communities. This level of detail offers an unprecedented opportunity for researchers and public health leaders to focus on where these differences are the most prominent, and possibly give us a clearer picture on what can be done about it.
These data raise many important questions, too. What can we learn from places that are doing well in caring for their most disadvantaged populations? How can these factors be sustained, replicated, and transferred to other places? Are there current policies and/or community services that contribute to or inhibit gaining access to appropriate clinical care, healthy and affordable food, good schools, and/or economic opportunities?
To help answer these questions, the GBD U.S. Health Disparities Collaborators at NIH, in partnership with IHME, have developed a comprehensive database and interactive data visualization tool that provides life expectancy and all-cause mortality by race and ethnicity for 3,110 U.S. counties from 2000-2019. Efforts are underway to expand the database to include causes of death and risk factors by race/ethnicity and education, as well as to disaggregate some of the major racial-ethnic groups.
Using IHME’s established model of comprehensive and replicable data collection, the joint effort aims to improve access to health data resources, bolster analytic approaches, and deliver user-friendly estimates to the wider research and health policy community. The collection’s standardized, comprehensive, historical, and real-time data can be the cornerstone for efforts to address disparities and advance health equity.
It is important to note that the Lancet study only included data from before the COVID-19 pandemic. The pandemic’s disproportionate effect on overall mortality and life expectancy has exacerbated existing health disparities. Disaggregated data are essential in helping to understand the underlying mechanisms of health disparities and guiding the development and implementation of interventions that address local needs.
As a clinician scientist, I have made a personal commitment at NIMHD to foster and encourage data collection with standardized measures, harmonization, and efficient data sharing to help us explore the nuances within all populations and their communities. Without these guiding principles for managing data, inequities remain unseen and unaddressed. Scientists, clinicians, and policymakers can all potentially benefit from this work if we use the data to inform our actions. It is an opportunity to implement real change in our NIH-wide combined efforts to reduce health disparities and improve quality of life and longevity for all populations.
Reference:
[1] Life expectancy by county, race, and ethnicity in the USA, 2000-19: a systematic analysis of health disparities. GBD US Health Disparities Collaborators. Lancet. 2022 Jul 2;400(10345):25-38.
Links:
Understand Health Disparities Series (National Institute on Minority Health and Health Disparities/NIH)
HD Pulse (NIMHD)
PhenX Social Determinants of Health Toolkit (NIMHD)
Institute for Health Metrics (University of Washington, Seattle)
NIH Support: The members of the GBD U.S. Health Disparities Collaborators at NIH include: National Heart, Lung, and Blood Institute; National Cancer Institute; National Institute on Aging; National Institute of Arthritis and Musculoskeletal and Skin Diseases; NIH Office of Disease Prevention; NIH Office of Behavioral and Social Science Research
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 17th in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
Unlocking Potential in The Next Generation of Scientists
Posted on by Griffin P. Rodgers, M.D., M.A.C.P., National Institute of Diabetes and Digestive and Kidney Diseases

While talent is everywhere, opportunity is not. That belief, and meeting people where they are, have been the impetus for the efforts of NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to nurture diverse research talent in the Pacific Islands. Most recently that effort manifested in opening a new biomedical research laboratory at Southern High School, located in Santa Rita village on the island of Guam.
One of seven research labs in the Pacific Islands established under NIDDK’s Short-Term Research Experience Program to Unlock Potential (STEP-UP), the facility provides research training to high school and college students from historically underserved populations, which is the mission of STEP-UP. The goal is to foster a diverse, talented scientific workforce.
Created by NIDDK more than 20 years ago, STEP-UP aims to make opportunities accessible to aspiring scientists nationwide, regardless of their background or zip code. In 2009, we expanded the program to the Pacific Islands. By working with academic and nonprofit coordinating centers throughout the United States and its Pacific territories, the program enables students to gain hands-on research experience, one-on-one mentorship, and access to modern laboratory techniques without travelling far from home.
For Mata’uitafa Solomona-Faiai, a Ph.D. student at Yale University School of Public Health, New Haven, CT, the exposure to science through STEP-UP turned into a passion for research. Solomona-Faiai participated in STEP-UP as a high schooler in American Samoa, and again as a college undergraduate. After getting her master’s degree at George Washington University in Washington, D.C., she returned to American Samoa to conduct epidemiology research—and became a co-mentor to high school STEP-UP students.
Her experiences in STEP-UP made her realize she wanted to pursue a life of public health research and gave her the skills to help pave that path. I was delighted to learn that Solomona-Faiai recently received an NIDDK Diversity Supplement to help support her research, which will focus on improving diabetes outcomes among adolescents from the Pacific Islands. She also hopes one day to run her own research group as an independent principal investigator, and I’m confident in her tenacity to make that happen!
Solomona-Faiai is among more than 2,300 students who have participated in STEP-UP since 2000. Her story embodies the scientific potential we can access if we contribute the right resources and tools. Early evaluation results of STEP-UP from 2002 to 2018 showed that many of the program’s participants have pursued careers as researchers, physicians, and physician-scientists [1]. In addition, of the more than 300 high school STEP-UP participants in the Pacific Islands, most have gone on to attend four-year universities, many majoring in STEM disciplines [2]. I’m heartened to know our efforts are paying off.
Bringing scientific opportunity to the Pacific Islands has entailed more than just placing students into research labs. We found we had to help create infrastructure—building labs in often under-resourced areas where nearly no biomedical infrastructure previously existed.
Since 2008, NIDDK has helped establish research labs at high schools and community colleges in the American Samoa, Commonwealth of the Northern Mariana Islands, Republic of the Marshall Islands, Federated States of Micronesia, Republic of Palau, and now Guam. The labs are also available to faculty to conduct their own science and to train as mentors. Having the support of their teachers is particularly important for students in these areas, many of whom have never heard of biomedical research before. For them, the labs often provide their first real exposure to science.
As proud as I am of the strides we’ve made, I know we have much more work to do. That’s why I’m grateful to the unwavering commitment of my colleagues, including Lawrence Agodoa who has pioneered STEP-UP and other programs in NIDDK’s Office of Minority Health Research Coordination; Robert Rivers, who coordinates NIDDK’s training programs; and George Hui at University of Hawaii at Manoa, who has directed the Pacific STEP-UP for 15 years.
They, like so many of NIDDK’s staff, partners, and grantees, will continue to work relentlessly to achieve our institute’s vision of developing a talented biomedical research workforce that fully represents the diverse fabric of the United States and its territories.
This month, we welcome a new class of STEP-UP participants, and I hope that, like Solomona-Faiai, they’ll experience the excitement of scientific discovery that will help shape their career goals and propel them to attain those goals. And I’m reminded of the tremendous responsibility we have to nurture and support the next generation of scientists. After all, the future of our nation’s health is in their hands.
References:
[1] NIDDK’s short-term research experience for underrepresented persons (STEP-UP) program. Rivers, R., Brinkley, K., Agodoa, L. JHDRP. 2019 Summer; 12: 1-2.
[2] Promoting local talents to fight local health issues: STEP-UP in the Pacific. Golshan, A., Hui, G. JHDRP. 2019 Summer; 12: 31-32.
Links:
Short-Term Research Experience Program to Unlock Potential (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Office of Minority Health Research Coordination (NIDDK)
Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 12th in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
Tuberculosis: An Ancient Disease in Need of Modern Scientific Tools
Posted on by Anthony S. Fauci, M.D., National Institute of Allergy and Infectious Diseases

Although COVID-19 has dominated our attention for the past two years, tuberculosis (TB), an ancient scourge, remains a dominating infectious disease globally, with an estimated 10 million new cases and more than 1.3 million deaths in 2020. TB disproportionately afflicts the poor and has long been the leading cause of death in people living with HIV.
Unfortunately, during the global COVID-19 pandemic, recent gains in TB control have been stalled or reversed. We’ve seen a massive drop in new TB diagnoses, reflecting poor access to care and an uptick in deaths in 2020 [1].
We are fighting TB with an armory of old weapons inferior to those we have for COVID-19. The Bacillus Calmette–Guérin (BCG) vaccine, the world’s only licensed TB vaccine, has been in use for more than 100 years. While BCG is somewhat effective at preventing TB meningitis in children, it provides more limited durable protection against pulmonary TB in children and adults. More effective vaccination strategies to prevent infection and disease, decrease relapse rates, and shorten durations of treatment are desperately needed to reduce the terrible global burden of TB.
In this regard, over the past five years, several exciting research advances have generated new optimism in the field of TB vaccinology. Non-human primate studies conducted at my National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center and other NIAID-funded laboratories have demonstrated that effective immunity against infection is achievable and that administering BCG intravenously, rather than under the skin as it currently is given, is highly protective [2].
Results from a phase 2 trial testing BCG revaccination in adolescents at high risk of TB infection suggested this approach could help prevent TB [3]. In addition, a phase 2 trial of an experimental TB vaccine based on the recombinant protein M72 and an immune-priming adjuvant, AS01, also showed promise in preventing active TB disease in latently infected adults [4].
Both candidates are now moving on to phase 3 efficacy trials. The encouraging results of these trials, combined with nine other candidates currently in phase 2 or 3 studies [5], offer new hope that improved vaccines may be on the horizon. The NIAID is working with a team of other funders and investigators to analyze the correlates of protection from these studies to inform future TB vaccine development.
Even with these exciting developments, it is critical to accelerate our efforts to enhance and diversify the TB vaccine pipeline by addressing persistent basic and translational research gaps. To this end, NIAID has several new programs. The Immune Protection Against Mtb Centers are taking a multidisciplinary approach to integrate animal and human data to gain a comprehensive understanding of the immune responses required to prevent TB infection and disease.
This spring, NIAID will fund awards under the Innovation for TB Vaccine Discovery program that will focus on the discovery and early evaluation of novel TB vaccine candidates with the goal of diversifying the TB vaccine pipeline. Later this year, the Advancing Vaccine Adjuvant Research for TB program will systematically assess combinations of TB immunogens and adjuvants. Finally, NIAID’s well-established clinical trials networks are planning two new clinical trials of TB vaccine candidates.
As we look to the future, we must apply the lessons learned in the development of the COVID-19 vaccines to longstanding public health challenges such as TB. COVID-19 vaccine development was hugely successful due to the use of novel vaccine platforms, structure-based vaccine design, community engagement for rapid clinical trial enrollment, real-time data sharing with key stakeholders, and innovative trial designs.
However, critical gaps remain in our armamentarium. These include the harnessing the immunology of the tissues that line the respiratory tract to design vaccines more adept at blocking initial infection and transmission, employing thermostable formulations and novel delivery systems for resource-limited settings, and crafting effective messaging around vaccines for different populations.
As we work to develop better ways to prevent, diagnose, and treat TB, we will do well to remember the great public health icon, Paul Farmer, who tragically passed away earlier this year at a much too young age. Paul witnessed firsthand the devastating consequences of TB and its drug resistant forms in Haiti, Peru, and other parts of the world.
In addition to leading efforts to improve how TB is treated, Paul provided direct patient care in underserved communities and demanded that the world do more to meet their needs. As we honor Paul’s legacy, let us accelerate our efforts to find better tools to fight TB and other diseases of global health importance that exact a disproportionate toll among the poor and underserved.
References:
[1] Global tuberculosis report 2021. WHO. October 14, 2021.
[2] Prevention of tuberculosis in macaques after intravenous BCG immunization. Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH,. Hughes TK, Pokkali S, Swanson PA, Grant NL, Rodgers MA, Kamath M, Causgrove CM, Laddy DJ, Bonavia A, Casimiro D, Lin PL, Klein E, White AG, Scanga CA, Shalek AK, Roederer M, Flynn JL, and Seder RA. Nature. 2020 Jan 1; 577: 95–102.
[3] Prevention of M. tuberculosis Infection with H4:IC31 vaccine or BCG revaccination. Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F,Bilek N., Mabwe S, Makhethe L, Erasmus M, Toefy A, Mulenga H, Hanekom WA, et al. N Engl J Med 2018; 379:138-149.
[4] Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, Scriba TJ, Akite EJ, Ayles HM, et al.
[5] Pipeline Report 2021: Tuberculosis Vaccines. TAG. October 2021.
Links:
Tuberculosis (National Institute of Allergy and Infectious Diseases/NIH)
NIAID Strategic Plan for Tuberculosis Research
Immune Mechanisms of Protection Against Mycobacterium tuberculosis Centers (IMPAc-TB) (NIAID)
Partners in Health (Boston, MA)
[Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the seventh in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.]
COVID-19 Infected Many More Americans in 2020 than Official Tallies Show
Posted on by Dr. Francis Collins
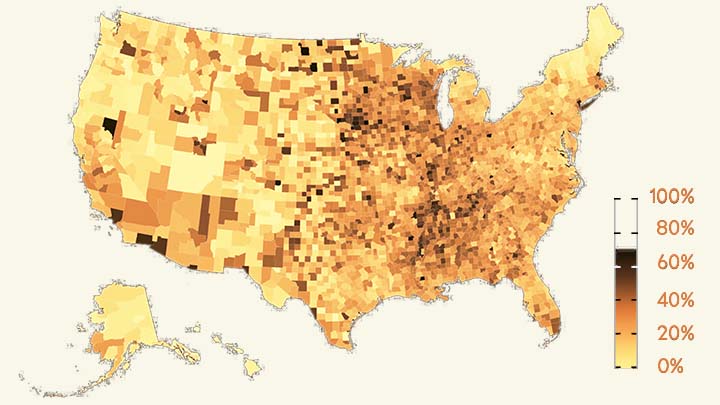
At the end of last year, you may recall hearing news reports that the number of COVID-19 cases in the United States had topped 20 million. While that number came as truly sobering news, it also likely was an underestimate. Many cases went undetected due to limited testing early in the year and a large number of infections that produced mild or no symptoms.
Now, a recent article published in Nature offers a more-comprehensive estimate that puts the true number of infections by the end of 2020 at more than 100 million [1]. That’s equal to just under a third of the U.S. population of 328 million. This revised number shows just how rapidly this novel coronavirus spread through the country last year. It also brings home just how timely the vaccines have been—and continue to be in 2021—to protect our nation’s health in this time of pandemic.
The work comes from NIH grantee Jeffrey Shaman, Sen Pei, and colleagues, Columbia University, New York. As shown above in the map, the researchers estimated the percentage of people who had been infected with SARS-CoV-2, the novel coronavirus that causes COVID-19, in communities across the country through December 2020.
To generate this map, they started with existing national data on the number of coronavirus cases (both detected and undetected) in 3,142 U.S. counties and major metropolitan areas. They then factored in data from the Centers for Disease Control and Prevention (CDC) on the number of people who tested positive for antibodies against SARS-CoV-2. These CDC data are useful for picking up on past infections, including those that went undetected.
From these data, the researchers calculated that only about 11 percent of all COVID-19 cases were confirmed by a positive test result in March 2020. By the end of the year, with testing improvements and heightened public awareness of COVID-19, the ascertainment rate (the number of infections that were known versus unknown) rose to about 25 percent on average. This measure also varied a lot across the country. For instance, the ascertainment rates in Miami and Phoenix were higher than the national average, while rates in New York City, Los Angeles, and Chicago were lower than average.
How many people were potentially walking around with a contagious SARS-CoV-2 infection? The model helps to answer this, too. On December 31, 2020, the researchers estimate that 0.77 percent of the U.S. population had a contagious infection. That’s about 1 in every 130 people on average. In some places, it was much higher. In Los Angeles, for example, nearly 1 in 40 (or 2.42 percent) had a SARS-CoV-2 infection as they rang in the New Year.
Over the course of the year, the fatality rate associated with COVID-19 dropped, at least in part due to earlier diagnosis and advances in treatment. The fatality rate went from 0.77 percent in April to 0.31 percent in December. While this is great news, it still shows that COVID-19 remains much more dangerous than seasonal influenza (which has a fatality rate of 0.08 percent).
Today, the landscape has changed considerably. Vaccines are now widely available, giving many more people immune protection without ever having to get infected. And yet, the rise of the Delta and other variants means that breakthrough infections and reinfections—which the researchers didn’t account for in their model—have become a much bigger concern.
Looking ahead to the end of 2021, Americans must continue to do everything they can to protect their communities from the spread of this terrible virus. That means getting vaccinated if you haven’t already, staying home and getting tested if you’ve got symptoms or know of an exposure, and taking other measures to keep yourself and your loved ones safe and well. These measures we take now will influence the infection rates and susceptibility to SARS-CoV-2 in our communities going forward. That will determine what the map of SARS-CoV-2 infections will look like in 2021 and beyond and, ultimately, how soon we can finally put this pandemic behind us.
Reference:
[1] Burden and characteristics of COVID-19 in the United States during 2020. Pei S, Yamana TK, Kandula S, Galanti M, Shaman J. Nature. 2021 Aug 26.
Links:
COVID-19 Research (NIH)
Sen Pei (Columbia University, New York)
Jeffrey Shaman (Columbia University, New York)
Following COVID-19 Vaccines Across the United States
Posted on by Dr. Francis Collins
Recently, there is a new and very hopeful COVID-19 number for everyone to track: the total number of vaccine doses that have been administered in the United States. If 80 percent of Americans roll up their sleeves in the coming months and accept COVID-19 vaccinations, we can greatly slow the spread of the novel coronavirus in our communities and bring this horrible pandemic to an end in 2021.
So far, more than 20 million people in our country have received one or two doses of either the Pfizer or Moderna vaccine. While this number is lower than initially projected for a variety of logistical reasons, we’re already seeing improvements in the distribution system that has made it possible to get close to 1 million doses administered per day.
If you want to keep track of the vaccine progress in your state over the coming weeks, it’s now pretty easy to do online. A fine resource is the vaccine information on the Centers for Disease Control and Prevention (CDC) COVID Data Tracker. It offers an interactive state-by-state map, as well as data on vaccinations in long-term care facilities. Keep in mind that there’s a delay of three to five days in reporting actual vaccinations from the states.
There’s also a lot of useful information on the Johns Hopkins Coronavirus Resource Center’s Vaccine Tracker. Posting the daily updates is a team, led by William Moss, that draws on the expertise of data scientists, analysts, programmers, and researchers. The Hopkins team gathers its vaccination data from each state’s official dashboard, webpages, press releases, or wherever cumulative numbers are reported. Not all states publish the same vaccine information, and that’s what can make the Vaccine Tracker so challenging to compile.
The Hopkins team now presents on its homepage the top 10 U. S. states and territories to vaccinate fully the highest percentage of their residents. With another click, there’s also a full rundown of vaccine administration by state and territory, plus the District of Columbia. The site also links to lots of other information about COVID-19—including cases, testing, contact tracing, and an interactive tool about vaccine development.
In uncertain times, knowledge can be a source of comfort. That’s what makes these interactive COVID-19 resources so helpful and empowering. They show that, with time, safe and effective COVID-19 vaccines will indeed coming to everyone. I hope that you will accept your vaccine, like I did when given the opportunity. However, until we get to the point where most Americans are immunized, we must stay vigilant and keep up our tried-and-true public health measures such as wearing masks, limiting physical interactions (especially indoors), and washing our hands.
Links:
COVID-19 Research (NIH)
CDC COVID Data Tracker (Centers for Disease Control and Prevention, Atlanta)
Coronavirus Resource Center (Johns Hopkins University School of Medicine)
William Moss (Johns Hopkins University, Baltimore)
International Vaccine Access Center (Johns Hopkins Bloomberg School of Public Health, Baltimore)
Next Page