pregnancy
Diagnosis and Treatment of Mental Health Conditions During and After Pregnancy is On the Rise, But Disparities Still Exist
Posted on by Dr. Monica M. Bertagnolli

Pregnancy and childbirth are often thought of as joyful times. Yet, we know that mental health conditions including perinatal depression, anxiety, and post-traumatic stress disorder (PTSD) are common complications during and after pregnancy, and this is contributing to a maternal health crisis in this country.
Now, a trio of NIH-supported studies reported in the journal Health Affairs show that diagnosis and treatment of mental health conditions such as anxiety, depression, and PTSD during pregnancy and in the first year after giving birth rose significantly in Americans with private health insurance from 2008 to 2020.1,2,3 While these are encouraging signs of increasing mental health awareness and service use, these studies also showed that this increase hasn’t happened equally across all demographic groups and states, making it clear there’s more work to do to ensure that people from all walks of life have access to the care they need, regardless of their race, ethnicity, geographic location, financial status, or other factors.
The findings come from a research team including Kara Zivin and Stephanie Hall and their colleagues at the University of Michigan, Ann Arbor. They recognized a worrying crisis in maternal mental illness in the U.S., with serious health risks and many potential long-term negative impacts for new parents and their infants. While earlier studies had looked at the prevalence of mental health conditions in the perinatal period, Zivin and Hall wanted to explore nationwide trends in the diagnosis of PTSD and what they refer to as perinatal mood and anxiety disorders (PMAD) among people giving birth between ages 15 and 44.
In the first study, using a database of administrative medical claims representing insured people across the U.S., the researchers examined PMAD diagnoses (including depressive and anxiety disorders) among those with private health insurance and found that the diagnosis rate had increased by more than 93% over the 12-year period under study. The rates also showed a sharp uptick after 2015, after the Affordable Care Act (ACA) went into effect in 2014. By 2020, 28% of those who were pregnant or in their postpartum period received a diagnosis of PMAD.
The researchers found that rate of suicidality (that is, suicidal ideation or diagnoses of self-harm) among people during pregnancy and just after childbirth more than doubled over the same period, although that rate dipped among those who had received a PMAD diagnosis. The rate at which individuals who were pregnant or in their postpartum period received any form of talk therapy covered by their private insurance increased by 16% from 2008 to 2020.
The second report focused specifically on PTSD diagnoses among privately insured people over the same period. The data show a near quadrupling of PTSD diagnoses in the months surrounding childbirth, with diagnoses in nearly 2% of privately insured people by 2020. Most of the increase in PTSD diagnoses occurred in people who had already been diagnosed with PMAD.
In the third study, the researchers wanted to learn if there were increases in antidepressant prescriptions for those diagnosed with PMAD, especially after new guidelines from several professional organizations were issued in 2015 and 2016. The findings show a decrease of 3% per year from 2008 to 2016, followed by a 32% increase in 2017, with prescription rates continuing to climb each year through 2020. By 2020, slightly less than half of those diagnosed with PMAD received a prescription for an antidepressant, suggesting that the clinical recommendations made a difference in clinical practice. However, there are signs of racial disparities, with White people with PMAD diagnoses receiving antidepressants more often than people in other racial groups.
In fact, all three studies show differences between people in different age, race, ethnicity, and geographic groups. For example, White people were more likely to be diagnosed with PTSD during the perinatal period, followed by Black people. By comparison, people of unknown race, as well as people who identified as Hispanic and Asian, were diagnosed less often. At the same time, the largest increase in PMAD diagnoses among races and ethnicities was among Black people, increasing from 14% of deliveries in 2008 to 22% in 2020.
Looking at age groups, the initial prevalence of PMAD diagnoses was highest among people aged 40 to 44, yet the youngest people (aged 15 to 24) experienced the largest increase in diagnoses. The youngest age group also had the largest increases in antidepressant prescriptions, and those aged 15 to 26 were more likely to be diagnosed with PTSD than those in older age groups. The first study also showed wide variation between states in the rate of PMAD diagnoses before and after implementation of the ACA.
The findings suggest there have been improvements in doctors’ recognition and treatment of PMAD and PTSD in the months surrounding childbirth. Increases in health insurance coverage and laws requiring equal treatment of mental health conditions may also be playing a role, along with greater social awareness and acceptance of mental health conditions generally. The researchers noted, however, that the findings don’t represent people with government-funded health insurance or those who lack health insurance completely.
It will be important to learn in future studies more about those who may still not be receiving the mental health care they need. The researchers report plans to look deeper into changes that have taken place at the state level and the impact of the pandemic and the rise of telehealth since 2020. Other recent NIH-supported research suggests that relatively straightforward interventions to reduce postpartum anxiety and depression can be remarkably effective. The key step will be not only identifying interventions that work, but also figuring out how to deliver effective treatments to the people who need them.
References:
[1] Zivin K, et al. Perinatal Mood and Anxiety Disorders Rose Among Privately Insured People, 2008-20. Health Affairs. DOI: 10.1377/hlthaff.2023.01437 (2024).
[2] Hall SV, et al. Perinatal Posttraumatic Stress Disorder Diagnoses Among Commercially Insured People Increased, 2008-20. Health Affairs. DOI: 10.1377/hlthaff.2023.01447 (2024).
[3] Hall SV, et al. Antidepressant Prescriptions Increased for Privately Insured People With Perinatal Mood And Anxiety Disorder, 2008-20. Health Affairs. DOI: 10.1377/hlthaff.2023.01448 (2024).
NIH Support: National Institute of Mental Health, National Institute on Minority Health and Health Disparities
Understanding Childbirth Through a Single-Cell Atlas of the Placenta
Posted on by Dr. Monica M. Bertagnolli

While every birth story is unique, many parents would agree that going into labor is an unpredictable process. Although most pregnancies last about 40 weeks, about one in every 10 infants in the U.S. are born before the 37th week of pregnancy, when their brain, lungs, and liver are still developing.1 Some pregnancies also end in an unplanned emergency caesarean delivery after labor fails to progress, for reasons that are largely unknown. Gaining a better understanding of what happens during healthy labor at term may help to elucidate why labor doesn’t proceed normally in some cases.
In a recent development, NIH scientists and their colleagues reported some fascinating new findings that could one day give healthcare providers the tools to better understand and perhaps even predict labor.2 The research team produced an atlas showing the patterns of gene activity that take place in various cell types during labor. To create the atlas, they examined tissues from the placentas of 18 patients not in labor who underwent caesarean delivery and 24 patients in labor. The researchers also analyzed blood samples from another cohort of more than 250 people who delivered at various timepoints. This remarkable study, published in Science Translational Medicine, is the first to analyze gene activity at the single-cell level to better understand the communication that occurs between maternal and fetal cells and tissues during labor.
The placenta is an essential organ for bringing nutrients and oxygen to a growing fetus. It also removes waste, provides immune protection, and supports fetal development. The placenta participates in the process of normal labor at term and preterm labor. Problems with the placenta can lead to many issues, including preterm birth. To create the placental atlas, the study team used an approach called single-cell RNA sequencing. Messenger RNA molecules transcribed or copied from DNA serve as templates for proteins, including those that send important signals between tissues. By sequencing RNAs at the single-cell level, it’s possible to examine gene activity and signaling patterns in many thousands of individual cells at once. This method allows scientists to capture and describe in detail the activities within individual cell types along with interactions among cells of different types and in immune or other key signaling pathways.
Using this approach, the researchers found that cells in the chorioamniotic membranes, which surround the fetus and rupture as part of the labor and delivery process, showed the greatest changes. They also found cells in the mother and fetus that were especially active in generating inflammatory signals. They note that these findings are consistent with previous research showing that inflammation plays an important role in sustaining labor.
Gene activity patterns and changes in the placenta can only be studied after the placenta is delivered. However, it would be ideal if these changes could be identified in the bloodstream of mothers earlier in pregnancy—before labor—so that health care providers can intervene if necessary. The recent study showed that this was possible: Certain gene activity patterns observed in placental cells during labor could be detected in blood tests of women earlier in pregnancy who would later go on to have a preterm birth. The authors note that more research is needed to validate these findings before they can be used as a clinical tool.
Overall, these findings offer important insight into the underlying biology that normally facilitates healthy labor and delivery. They also offer preliminary proof-of-concept evidence that placental biomarkers present in the bloodstream during pregnancy may help to identify pregnancies at increased risk for preterm birth. While much more work and larger studies are needed, these findings suggest that it may one day be possible to identify those at risk for a difficult or untimely labor, when there is still opportunity to intervene.
The research was conducted by the Pregnancy Research Branch part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and led by Roberto Romero, M.D., D.Med.Sci., NICHD; Nardhy Gomez-Lopez, Ph.D., Washington University School of Medicine in St. Louis; and Roger Pique-Regi, Ph.D., Wayne State University, Detroit.
References:
[1] Preterm Birth. CDC.
[2] Garcia-Flores V, et al., Deciphering maternal-fetal crosstalk in the human placenta during parturition using single-cell RNA sequencing. Science Translational Medicine DOI: 10.1126/scitranslmed.adh8335 (2024).
NIH Support: Eunice Kennedy Shriver National Institute of Child Health and Human Development
Visualizing The Placenta, a Critical but Poorly Understood Organ
Posted on by Diana W. Bianchi, M.D., Eunice Kennedy Shriver National Institute of Child Health and Human Development
The placenta is the Rodney Dangerfield of organs; it gets no respect, no respect at all. This short-lived but critical organ supports pregnancy by bringing nutrients and oxygen to the fetus, removing waste, providing immune protection, and producing hormones to support fetal development.
It also influences the lifelong health of both mother and child. Problems with the placenta can lead to preeclampsia, gestational diabetes, poor fetal growth, preterm birth, and stillbirth. Although we were all connected to one, the placenta is the least understood, and least studied, of all human organs.
What we do know about the human placenta largely comes from studying it after delivery. But that’s like studying the heart after it’s stopped beating. It doesn’t help us predict complications in time to avert a crisis.
To fill these knowledge gaps, NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) developed the Human Placenta Project (HPP) to noninvasively study the placenta during pregnancy. Since 2014, this approximately $88 million collaborative research effort has been developing ultrasound, magnetic resonance imaging (MRI), and blood-based biomarker methods to study how the placenta functions in real time and in greater detail.
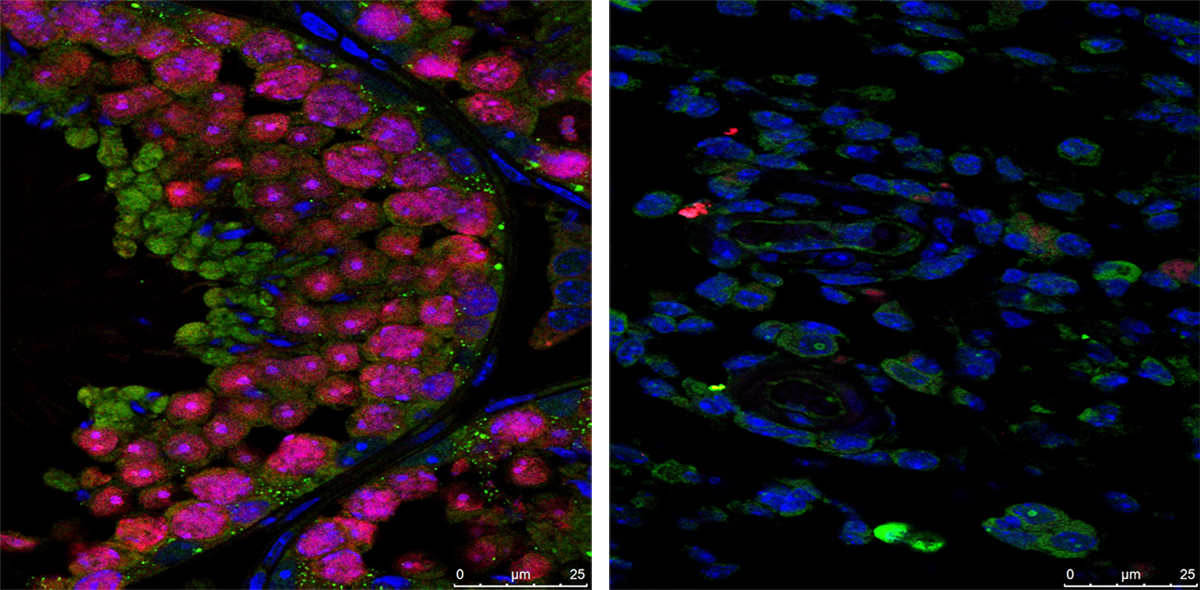
As illustrated in the image above, advanced ultrasound tools allowed HPP researchers at Eastern Virginia Medical School, Norfolk, and the University of Texas Medical Branch, Galveston, to gain a detailed look at the placenta’s intricate arrangement of blood vessels, or vasculature. By evaluating both fetal (left panel) and maternal (right panel) placental vasculature in 610 pregnant people starting at 13 weeks of gestation, the investigators aimed to identify early changes that predicted later complications.
They observed that such changes can start in the first trimester and affect both the vasculature and placental tissue. While further research is needed, these findings suggest that placental ultrasound monitoring can inform efforts to prevent and treat pregnancy complications.
Another HPP team led by Boston Children’s Hospital is developing an MRI strategy to monitor blood flow and oxygen transport through the placenta during pregnancy. Interpreting and visualizing MRI data of the placenta is challenging because of its variable shape, the tendency of muscles in the uterus to begin tightening or contracting well before labor [1], and other factors.
As shown in the video above, the researchers developed a way to account for the motion of the uterus and “freeze” the placenta to make it easier to study (left two panels of video) [2]. They also developed algorithms to better visualize the complex patterns of placental oxygen content during contractions (center panel) [3]. The scientists then carried out initial visualizations of blood flow through the placenta shortly after delivery (second panel from right) [4].
They now intend to map these MRI findings to the placenta itself after delivery (far right panel), which will allow them to explore how additional factors such as gene expression patterns and genetic variants contribute to placental function. Ultimately, they plan to apply these MRI techniques to monitor the placenta in real time during pregnancy and identify changes that indicate compromised function early enough to adjust maternal management as needed.
Other HPP efforts focus on identifying components in maternal blood that reflect the status of the placenta. For example, an HPP research team led by scientists at the University of California, Los Angeles, adapted non-invasive prenatal testing methods to analyze genetic material shed from the placenta into the maternal bloodstream. Their findings suggest that distinctive patterns in this genetic material detected early in pregnancy may indicate risk for later complications [5].
Another HPP team, led by investigators at Columbia University, New York, helped establish that extracellular RNAs (exRNAs) released by the placenta into maternal circulation reflect the placenta’s status at a cellular level beginning in the first trimester. To harness the potential of exRNA biomarkers, the investigators are optimizing methods to isolate, sequence, and analyze exRNAs in maternal blood.
These are just a few examples of the cutting-edge work being funded through the HPP, which complements NICHD’s longstanding investment in basic research to unravel the physiology of and real-time gene expression in the placenta. Unlocking the secrets of the placenta may one day help us to prevent and treat a range of common pregnancy complications, while also providing insights into other areas of science and medicine such as cardiovascular disease and aging. NICHD is committed to giving this important organ the respect it deserves.
References:
[1] Placental MRI: Effect of maternal position and uterine contractions on placental BOLD MRI measurements. Abaci Turk E, Abulnaga SM, Luo J, Stout JN, Feldman H, Turk A, Gagoski B, Wald LL, Adalsteinsson E, Roberts DJ, Bibbo C, Robinson JN, Golland P, Grant PE, Barth, Jr WH. Placenta. 2020 Jun 1; 95: 69-77.
[2] Spatiotemporal alignment of in utero BOLD-MRI series. Turk EA, Luo J, Gagoski B, Pascau J, Bibbo C, Robinson JN, Grant PE, Adalsteinsson E, Golland P, Malpica N. J Magn Reson Imaging. 2017 Aug;46(2):403-412.
[3] Volumetric parameterization of the placenta to a flattened template. Abulnaga SM, Turk EA, Bessmeltsev M, Grant PE, Solomon J, Golland P. IEEE transactions on medical imaging. 2022 April;41(4):925-936.
[4] Placental MRI: development of an MRI compatible ex vivo system for whole placenta dual perfusion. Stout JN, Rouhani S, Turk EA, Ha CG, Luo J, Rich K, Wald LL, Adalsteinsson E, Barth, Jr WH, Grant PE, Roberts DJ. Placenta. 2020 Nov 1; 101: 4-12.
[5] Cell-free DNA methylation and transcriptomic signature prediction of pregnancies with adverse outcomes. Del Vecchio G, Li Q, Li W, Thamotharan S, Tosevska A, Morselli M, Sung K, Janzen C, Zhou X, Pellegrini M, Devaskar SU. Epigenetics. 2021 Jun;16(6):642-661.
Links:
Human Placenta Project (Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH)
Preeclampsia (NICHD)
Understanding Gestational Diabetes (NICHD)
Preterm Labor and Birth (NICHD)
Stillbirth (NICHD)
Abuhamad Project Information (NIH RePORTER)
Grant Project Information (NIH RePORTER)
Devaskar Project Information (NIH RePORTER)
Williams Project Information (NIH RePORTER)
Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 10th in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
Studies Confirm COVID-19 mRNA Vaccines Safe, Effective for Pregnant Women
Posted on by Dr. Francis Collins
Clinical trials have shown that COVID-19 vaccines are remarkably effective in protecting those age 12 and up against infection by the coronavirus SARS-CoV-2. The expectation was that they would work just as well to protect pregnant women. But because pregnant women were excluded from the initial clinical trials, hard data on their safety and efficacy in this important group has been limited.
So, I’m pleased to report results from two new studies showing that the two COVID-19 mRNA vaccines now available in the United States appear to be completely safe for pregnant women. The women had good responses to the vaccines, producing needed levels of neutralizing antibodies and immune cells known as memory T cells, which may offer more lasting protection. The research also indicates that the vaccines might offer protection to infants born to vaccinated mothers.
In one study, published in JAMA [1], an NIH-supported team led by Dan Barouch, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, wanted to learn whether vaccines would protect mother and baby. To find out, they enrolled 103 women, aged 18 to 45, who chose to get either the Pfizer/BioNTech or Moderna mRNA vaccines from December 2020 through March 2021.
The sample included 30 pregnant women,16 women who were breastfeeding, and 57 women who were neither pregnant nor breastfeeding. Pregnant women in the study got their first dose of vaccine during any trimester, although most got their shots in the second or third trimester. Overall, the vaccine was well tolerated, although some women in each group developed a transient fever after the second vaccine dose, a common side effect in all groups that have been studied.
After vaccination, women in all groups produced antibodies against SARS-CoV-2. Importantly, those antibodies neutralized SARS-CoV-2 variants of concern. The researchers also found those antibodies in infant cord blood and breast milk, suggesting that they were passed on to afford some protection to infants early in life.
The other NIH-supported study, published in the journal Obstetrics & Gynecology, was conducted by a team led by Jeffery Goldstein, Northwestern’s Feinberg School of Medicine, Chicago [2]. To explore any possible safety concerns for pregnant women, the team took a first look for any negative effects of vaccination on the placenta, the vital organ that sustains the fetus during gestation.
The researchers detected no signs that the vaccines led to any unexpected damage to the placenta in this study, which included 84 women who received COVID-19 mRNA vaccines during pregnancy, most in the third trimester. As in the other study, the team found that vaccinated pregnant women showed a robust response to the vaccine, producing needed levels of neutralizing antibodies.
Overall, both studies show that COVID-19 mRNA vaccines are safe and effective in pregnancy, with the potential to benefit both mother and baby. Pregnant women also are more likely than women who aren’t pregnant to become severely ill should they become infected with this devastating coronavirus [3]. While pregnant women are urged to consult with their obstetrician about vaccination, growing evidence suggests that the best way for women during pregnancy or while breastfeeding to protect themselves and their families against COVID-19 is to roll up their sleeves and get either one of the mRNA vaccines now authorized for emergency use.
References:
[1] Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, Chandrashekar A, Patel S, Apraku Bondzie E, Sellers D, Barrett J, Sanborn O, Wan H, Chang A, Anioke T, Nkolola J, Bradshaw C, Jacob-Dolan C, Feldman J, Gebre M, Borducchi EN, Liu J, Schmidt AG, Suscovich T, Linde C, Alter G, Hacker MR, Barouch DH. JAMA. 2021 May 13.
[2] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: Measures of immunity and placental histopathology. Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Obstet Gynecol. 2021 May 11.
[3] COVID-19 vaccines while pregnant or breastfeeding. Centers for Disease Control and Prevention.
Links:
COVID-19 Research (NIH)
Barouch Laboratory (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston)
Jeffery Goldstein (Northwestern University Feinberg School of Medicine, Chicago)
NIH Support: National Institute of Allergy and Infectious Diseases; National Cancer Institute, National Institute of Child Health and Human Development; National Center for Advancing Translational Sciences; National Institute of Biomedical Imaging and Bioengineering
Vast Majority of Pregnant Women with COVID-19 Won’t Have Complications, Study Finds
Posted on by Dr. Francis Collins

It’s natural and highly appropriate for women to be concerned about their health and the wellbeing of their unborn babies during pregnancy. With the outbreak of the pandemic, those concerns have only increased, especially after a study found last spring that about 30 percent of pregnant women who become infected with SARS-CoV-2, the coronavirus that causes COVID-19, needed to be hospitalized [1].
But that early study didn’t clearly divide out hospitalizations that were due to pregnancy from those owing to complications of COVID-19. Now, a large, observational study has taken a more comprehensive look at the issue and published some reassuring news for parents-to-be: the vast majority of women who test positive for COVID-19 during their pregnancies won’t develop serious health complications [2]. What’s more, it’s also unlikely that their newborns will become infected with SARS-CoV-2.
The findings reported in JAMA Network Open come from a busy prenatal clinic that serves women who are medically indigent at Parkland Health and Hospital System, affiliated with the University of Texas Southwestern, Dallas. Researchers there, led by obstetrician Emily Adhikari, followed more than 3,300 pregnant women, most of whom were Hispanic (75 percent) or African American (14 percent). From March through August of this year, 252 women tested positive for COVID-19 during their pregnancies.
At diagnosis, 95 percent were asymptomatic or had only mild symptoms. Only 13 of the 252 COVID-19-positive women (5 percent) in the study developed severe or critical pneumonia, including just six with no or mild symptoms initially. Only 14 women (6 percent) were admitted to the hospital for management of their COVID-19 pneumonia, and all survived.
By comparing mothers with and without COVID-19 during pregnancy, the researchers found there was no increase in adverse pregnancy-related outcomes. Overall, women with COVID-19 during pregnancy were not more likely to give birth early on average. They weren’t at increased risk of dangerous preeclampsia, a pregnancy complication characterized by high blood pressure and organ damage, or an emergency C-section to protect the baby.
The researchers found no evidence that the placenta was compromised in any way by the SARS-CoV-2 infection. In most cases, newborns didn’t get sick. Only 6 of 188 infants (3 percent) tested positive for COVID-19. Most of those infected were born to mothers who were asymptomatic or had only mild illness.
This is all encouraging news. However, it is worth noting that mothers who developed severe COVID-19 before reaching 37 weeks, or well into the third trimester of pregnancy, were more likely to give birth prematurely. More research is needed, but the study also suggests that diabetes may increase the risk for severe COVID-19 in pregnancy.
This study’s bottom line is that most women who become infected with SARS-CoV-2 during pregnancy will do just fine. That doesn’t mean, however, that anyone should take this situation casually. The finding that 5 percent of pregnant women may become severely ill is still cause for concern. Plus not all researchers come to the same conclusion—an update to the first study cited in this post recently found a greater risk for pregnant women becoming severely ill from COVID-19 and giving birth prematurely.
Taken together, while there’s no need to panic about COVID-19 infection during pregnancy, it’s still a good idea for pregnant women and their loved ones to take extra precautions to protect their health. And, of course, follow the three W’s: Wear a mask, Watch your distance, and Wash your hands.
References:
[1] Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 27;69(12):343-346.
[2] Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, Spong CY. JAMA Netw Open. 2020 Nov 2;3(11):e2029256.
Links:
Coronavirus (COVID) (NIH)
Combat COVID (U.S. Department of Health and Human Services, Washington, D.C.)
Data on COVID-19 during Pregnancy: Severity of Maternal Illness (Centers for Disease Control and Prevent, Atlanta)
COVID-19 Treatment Guidelines: Special Considerations in Pregnancy (NIH)
Emily Adhikari (University of Texas Southwestern Medical Center, Dallas)
Fundamental Knowledge of Microbes Shedding New Light on Human Health
Posted on by Dr. Francis Collins

Basic research in biology generates fundamental knowledge about the nature and behavior of living systems. It is generally impossible to predict exactly where this line of scientific inquiry might lead, but history shows that basic science almost always serves as the foundation for dramatic breakthroughs that advance human health. Indeed, many important medical advances can be traced back to basic research that, at least at the outset, had no clear link at all to human health.
One exciting example of NIH-supported basic research is the Human Microbiome Project (HMP), which began 12 years ago as a quest to use DNA sequencing to identify and characterize the diverse collection of microbes—including trillions of bacteria, fungi, and viruses—that live on and in the healthy human body.
The HMP researchers have subsequently been using those vast troves of fundamental data as a tool to explore how microbial communities interact with human cells to influence health and disease. Today, these explorers are reporting their latest findings in a landmark set of papers in the Nature family of journals. Among other things, these findings shed new light on the microbiome’s role in prediabetes, inflammatory bowel disease, and preterm birth. The studies are part of the Integrative Human Microbiome Project.
If you’d like to keep up on the microbiome and other basic research journeys, here’s a good way to do so. Consider signing up for basic research updates from the NIH Director’s Blog and NIH Research Matters. Here’s how to do it: Go to Email Updates, type in your email address, and enter. That’s it. If you’d like to see other update possibilities, including clinical and translational research, hit the “Finish” button to access Subscriber Preferences.
As for the recent microbiome findings, let’s start with the prediabetes study [1]. An estimated 1 in 3 American adults has prediabetes, detected by the presence of higher than normal fasting blood glucose levels. If uncontrolled and untreated, prediabetes can lead to the more-severe type 2 diabetes (T2D) and its many potentially serious side effects [2].
George Weinstock, The Jackson Laboratory for Genomic Medicine, Farmington, CT, Michael Snyder, Stanford University, Palo Alto, CA, and colleagues report that they have assembled a rich new data set covering the complex biology of prediabetes. That includes a comprehensive analysis of the human microbiome in prediabetes.
The data come from monitoring the health of 106 people with and without prediabetes for nearly four years. The researchers met with participants every three months, drawing blood, assessing the gut microbiome, and performing 51 laboratory tests. All this work generated millions of molecular and microbial measurements that provided a unique biological picture of prediabetes.
The picture showed specific interactions between cells and microbes that were different for people who are sensitive to insulin and those whose cells are resistant to it (as is true of many of those with prediabetes). The data also pointed to extensive changes in the microbiome during respiratory viral infections. Those changes showed clear differences in people with and without prediabetes. Some aspects of the immune response also appeared abnormal in people who were prediabetic.
As demonstrated in a landmark NIH study several years ago [2], people with prediabetes can do a lot to reduce their chances of developing T2D, such as exercising, eating healthy, and losing a modest amount of body weight. But this study offers some new leads to define the biological underpinnings of T2D in its earliest stages. These insights potentially point to high value targets for slowing or perhaps stopping the systemic changes that drive the transition from prediabetes to T2D.
The second study features the work of the Inflammatory Bowel Disease Multi’omics Data team. It’s led by Ramnik Xavier and Curtis Huttenhower, Broad Institute of MIT and Harvard, Cambridge, MA. [4]
Inflammatory bowel disease (IBD) is an umbrella term for chronic inflammations of the body’s digestive tract, such as Crohn’s disease and ulcerative colitis. These disorders are characterized by remissions and relapses, and the most severe flares can be life-threatening. Xavier, Huttenhower, and team followed 132 people with and without IBD for a year, collecting samples of their gut microbiomes every other week along with biopsies and blood samples for a total of nearly 3,000 samples.
By integrating DNA, RNA, protein, and metabolic analyses, they followed precisely which microbial species were present. They could also track which biochemical functions those microbes were capable of performing, and which functions they actually were performing over the course of the study.
These data now offer the most comprehensive view yet of functional imbalances associated with changes in the microbiome during IBD flares. These data also show how those imbalances may be altered when a person with IBD goes into remission. It’s also noteworthy that participants completed questionnaires on their diet. This dataset is the first to capture associations between diet and the gut microbiome in a relatively large group of people over time.
The evidence showed that the gut microbiomes of people with IBD were significantly less stable than the microbiomes of those without IBD. During IBD activity, the researchers observed increases in certain groups of microbes at the expense of others. Those changes in the microbiome also came with other telltale metabolic and biochemical disruptions along with shifts in the functioning of an individual’s immune system. The shifts, however, were not significantly associated with people taking medications or their social status.
By presenting this comprehensive, “multi-omic” view on the microbiome in IBD, the researchers were able to single out a variety of new host and microbial features that now warrant further study. For example, people with IBD had dramatically lower levels of an unclassified Subdoligranulum species of bacteria compared to people without the condition.
The third study features the work of The Vaginal Microbiome Consortium (VMC). The study represents a collaboration between Virginia Commonwealth University, Richmond, and Global Alliance to Prevent Prematurity and Stillbirth (GAPPS). The VMC study is led by Gregory Buck, Jennifer Fettweis, Jerome Strauss,and Kimberly Jefferson of Virginia Commonwealth and colleagues.
In this study, part of the Multi-Omic Microbiome Study: Pregnancy Initiative, the team followed up on previous research that suggested a potential link between the composition of the vaginal microbiome and the risk of preterm birth [5]. The team collected various samples from more than 1,500 pregnant women at multiple time points in their pregnancies. The researchers sequenced the complete microbiomes from the vaginal samples of 45 study participants, who gave birth prematurely and 90 case-matched controls who gave birth to full-term babies. Both cases and controls were primarily of African ancestry.
Those data reveal unique microbial signatures early in pregnancy in women who went on to experience a preterm birth. Specifically, women who delivered their babies earlier showed lower levels of Lactobacillus crispatus, a bacterium long associated with health in the female reproductive tract. Those women also had higher levels of several other microbes. The preterm birth-associated signatures also were associated with other inflammatory molecules.
The findings suggest a link between the vaginal microbiome and preterm birth, and raise the possibility that a microbiome test, conducted early in pregnancy, might help to predict a woman’s risk for preterm birth. Even more exciting, this might suggest a possible way to modify the vaginal microbiome to reduce the risk of prematurity in susceptible individuals.
Overall, these landmark HMP studies add to evidence that our microbial inhabitants have important implications for many aspects of our health. We are truly a “superorganism.” In terms of the implications for biomedicine, this is still just the beginning of what is sure to be a very exciting journey.
References:
[1] Longitudinal multi-omics of host-microbe dynamics in prediabetes. Zhou W, Sailani MR, Contrepois K, Sodergren E, Weinstock GM, Snyder M, et. al. Nature. 2019 May 29.
[2] National Diabetes Statistics Report, 2017, Center for Disease Control and Prevention (Atlanta, GA)
[3] Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Diabetes Prevention Program Research Group.Lancet Diabetes Endocrinol.2015 Nov;3(11):866-875.
[4] Multi-omics of the gut microbial ecosystem in inflammatory bowel disease. Lloyd-Price J, Arze C. Ananthakrishnan AN, Vlamakis H, Xavier RJ, Huttenhower C, et. al. Nature. 2019 May 29.
[5] The vaginal microbiome and preterm birth. Fettweis JM, Serrano MG, Brooks, JP, Jefferson KK, Strauss JF, Buck GA, et al. Nature Med. 2019 May 29.
Links:
Insulin Resistance & Prediabetes (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Crohn’s Disease (NIDDK/NIH)
Ulcerative colitis (NIDDK/NIH)
Preterm Labor and Birth: Condition Information (Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH)
Global Alliance to Prevent Prematurity and Stillbirth (Seattle, WA)
NIH Integrative Human Microbiome Project
NIH Support:
Prediabetes Study: Common Fund; National Institute of Dental and Craniofacial Research; National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of Human Genome Research; National Center for Advancing Translational Sciences
Inflammatory Bowel Disease Study: Common Fund; National Institute of Diabetes and Digestive and Kidney Diseases; National Center for Advancing Translational Sciences; National Institute of Human Genome Research; National Institute of Dental and Craniofacial Research
Preterm Birth Study: Common Fund; National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development
Preeclampsia: Study Highlights Need for More Effective Treatment, Prevention
Posted on by Dr. Francis Collins

Thinkstock
It’s well known that preeclampsia, a condition characterized by a progressive rise in a pregnant woman’s blood pressure and appearance of protein in the urine, can have negative, even life-threatening impacts on the health of both mother and baby. Now, NIH-funded researchers have documented that preeclampsia is also taking a very high toll on our nation’s economic well-being. In fact, their calculations show that, in 2012 alone, preeclampsia-related care cost the U.S. health care system more than $2 billion.
These findings are especially noteworthy because preeclampsia rates in the United States have been steadily rising over the past 30 years, fueled in part by increases in average maternal age and weight. This highlights the urgent need for more research to develop new and more effective strategies to protect the health of all mothers and their babies.
Morning Sickness Associated with Lower Miscarriage Risk
Posted on by Dr. Francis Collins

Thinkstock
During the first trimester of pregnancy, many women experience what’s commonly known as “morning sickness.” As distressing as this nausea and vomiting can be, a team of NIH researchers has gathered some of the most convincing evidence to date that such symptoms may actually be a sign of something very positive: a lower risk of miscarriage.
In fact, when the researchers studied a group of women who had suffered one or two previous miscarriages, they found that the women who felt nauseous during their subsequent pregnancies were 50 to 75 percent less likely to miscarry than those without nausea. While it’s not yet exactly clear what’s going on, the findings lend support to the notion that morning sickness may arise from key biological factors that reflect an increased likelihood of a successful pregnancy.
Next Page