neural circuits
Experiencing the Neural Symphony Underlying Memory through a Blend of Science and Art
Posted on by John Ngai, PhD, NIH BRAIN Initiative
Ever wonder how you’re able to remember life events that happened days, months, or even years ago? You have your hippocampus to thank. This essential area in the brain relies on intense and highly synchronized patterns of activity that aren’t found anywhere else in the brain. They’re called “sharp-wave ripples.”
These dynamic ripples have been likened to the brain version of an instant replay, appearing most commonly during rest after a notable experience. And, now, the top video winner in this year’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative’s annual Show Us Your BRAINs! Photo and Video Contest allows you to witness the “chatter” that those ripples set off in other neurons. The details of this chatter determine just how durable a particular memory is in ways neuroscientists are still working hard to understand.
Neuroscientist Saman Abbaspoor in the lab of Kari Hoffman at Vanderbilt University, Nashville, in collaboration with Tyler Sloan from the Montreal-based Quorumetrix Studio, sets the stage in the winning video by showing an electrode or probe implanted in the brain that can reach the hippocampus. This device allows the Hoffman team to wirelessly record neural activity in different layers of the hippocampus as the animal either rests or moves freely about.
In the scenes that follow, neurons (blue, cyan, and yellow) flash on and off. The colors highlight the fact that this brain area and the neurons within it aren’t all the same. Various types of neurons are found in the brain area’s different layers, some of which spark the activity you see, while others dampen it.
Hoffman explains that the specific shapes of individual cells pictured are realistic but also symbolic. While they didn’t trace the individual branches of neurons in the brain in their studies, they relied on information from previous anatomical studies, overlaying their intricate forms with flashing bursts of activity that come straight from their recorded data.
Sloan then added yet another layer of artistry to the experience with what he refers to as sonification, or the use of music to convey information about the dynamic and coordinated bursts of activity in those cells. At five seconds in, you hear the subtle flutter of a sharp-wave ripple. With each burst of active neural chatter that follows, you hear the dramatic plink of piano keys.
Together, their winning video creates a unique sensory experience that helps to explain what goes on during memory formation and recall in a way that words alone can’t adequately describe. Through their ongoing studies, Hoffman reports that they’ll continue delving even deeper into understanding these intricate dynamics and their implications for learning and memory. Ultimately, they also want to explore how brain ripples, and the neural chatter they set off, might be enhanced to make memory formation and recall even stronger.
References:
S Abbaspoor & KL Hoffman. State-dependent circuit dynamics of superficial and deep CA1 pyramidal cells in macaques. BioRxiv DOI: 10.1101/2023.12.06.570369 (2023). Please note that this article is a pre-print and has not been peer-reviewed.
NIH Support: The NIH BRAIN Initiative
This article was updated on Dec. 15, 2023 to reflect better the collaboration on the project among Abbaspoor, Hoffman and Sloan.
How Neurons Make Connections
Posted on by Lawrence Tabak, D.D.S., Ph.D.

For many people, they are tiny pests. These fruit flies that sometimes hover over a bowl of peaches or a bunch of bananas. But for a dedicated community of researchers, fruit flies are an excellent model organism and source of information into how neurons self-organize during the insect’s early development and form a complex, fully functioning nervous system.
That’s the scientific story on display in this beautiful image of a larval fruit fly’s developing nervous system. Its subtext is: fundamental discoveries in the fruit fly, known in textbooks as Drosophila melanogaster, provide basic clues into the development and repair of the human nervous system. That’s because humans and fruit flies, though very distantly related through the millennia, still share many genes involved in their growth and development. In fact, 60 percent of the Drosophila genome is identical to ours.
Once hatched, as shown in this image, a larval fly uses neurons (magenta) to sense its environment. These include neurons that sense the way its body presses against the surrounding terrain, as needed to coordinate the movements of its segmented body parts and crawl in all directions.
This same set of neurons will generate painful sensations, such as the attack of a parasitic wasp. Paintbrush-like neurons in the fly’s developing head (magenta, left side) allow the insect to taste the sweetness of a peach or banana.
There is a second subtype of neurons, known as proprioceptors (green). These neurons will give the young fly its “sixth sense” understanding about where its body is positioned in space. The complete collection of developing neurons shown here are responsible for all the fly’s primary sensations. They also send these messages on to the insect’s central nervous system, which contains thousands of other neurons that are hidden from view.
Emily Heckman, now a postdoctoral researcher at the Michigan Neuroscience Institute, University of Michigan, Ann Arbor, captured this image during her graduate work in the lab of Chris Doe, University of Oregon, Eugene. For her keen eye, she received a trainee/early-career BioArt Award from the Federation of American Societies for Experimental Biology (FASEB), which each year celebrates the art of science.
The image is one of many from a much larger effort in the Doe lab that explores the way neurons that will partner find each other and link up to drive development. Heckman and Doe also wanted to know how neurons in the developing brain interconnect into integrated neural networks, or circuits, and respond when something goes wrong. To find out, they disrupted sensory neurons or forced them to take alternate paths and watched to see what would happen.
As published in the journal eLife [1], the system has an innate plasticity. Their findings show that developing sensory neurons instruct one another on how to meet up just right. If one suddenly takes an alternate route, its partner can still reach out and make the connection. Once an electrically active neural connection, or synapse, is made, the neural signals themselves slow or stop further growth. This kind of adaptation and crosstalk between neurons takes place only during a particular critical window during development.
Heckman says part of what she enjoys about the image is how it highlights that many sensory neurons develop simultaneously and in a coordinated process. What’s also great about visualizing these events in the fly embryo is that she and other researchers can track many individual neurons from the time they’re budding stem cells to when they become a fully functional and interconnected neural circuit.
So, the next time you see fruit flies hovering in the kitchen, just remember there’s more to their swarm than you think. Our lessons learned studying them will help point researchers toward new ways in people to restore or rebuild neural connections after devastating disruptions from injury or disease.
Reference:
Presynaptic contact and activity opposingly regulate postsynaptic dendrite outgrowth. Heckman EL, Doe CQ. Elife. 2022 Nov 30;11:e82093.
Links:
Research Organisms (National Institute of General Medical Sciences/NIH)
Doe Lab (University of Oregon, Eugene)
Emily Heckman (University of Michigan, Ann Arbor)
BioArt Awards (Federation of American Societies for Experimental Biology, Rockville, MD)
NIH Support: Eunice Kennedy Shriver National Institute of Child Health and Human Development
Tapping Into The Brain’s Primary Motor Cortex
Posted on by Dr. Francis Collins
If you’re like me, you might catch yourself during the day in front of a computer screen mindlessly tapping your fingers. (I always check first to be sure my mute button is on!) But all that tapping isn’t as mindless as you might think.
While a research participant performs a simple motor task, tapping her fingers together, this video shows blood flow within the folds of her brain’s primary motor cortex (gray and white), which controls voluntary movement. Areas of high brain activity (yellow and red) emerge in the omega-shaped “hand-knob” region, the part of the brain controlling hand movement (right of center) and then further back within the primary somatic cortex (which borders the motor cortex toward the back of the head).
About 38 seconds in, the right half of the video screen illustrates that the finger tapping activates both superficial and deep layers of the primary motor cortex. In contrast, the sensation of a hand being brushed (a sensory task) mostly activates superficial layers, where the primary sensory cortex is located. This fits with what we know about the superficial and deep layers of the hand-knob region, since they are responsible for receiving sensory input and generating motor output to control finger movements, respectively [1].
The video showcases a new technology called zoomed 7T perfusion functional MRI (fMRI). It was an entry in the recent Show Us Your BRAINs! Photo and Video Contest, supported by NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative.
The technology is under development by an NIH-funded team led by Danny J.J. Wang, University of Southern California Mark and Mary Stevens Neuroimaging and Informatics Institute, Los Angeles. Zoomed 7T perfusion fMRI was developed by Xingfeng Shao and brought to life by the group’s medical animator Jim Stanis.
Measuring brain activity using fMRI to track perfusion is not new. The brain needs a lot of oxygen, carried to it by arteries running throughout the head, to carry out its many complex functions. Given the importance of oxygen to the brain, you can think of perfusion levels, measured by fMRI, as a stand-in measure for neural activity.
There are two things that are new about zoomed 7T perfusion fMRI. For one, it uses the first ultrahigh magnetic field imaging scanner approved by the Food and Drug Administration. The technology also has high sensitivity for detecting blood flow changes in tiny arteries and capillaries throughout the many layers of the cortex [2].
Compared to previous MRI methods with weaker magnets, the new technique can measure blood flow on a fine-grained scale, enabling scientists to remove unwanted signals (“noise”) such as those from surface-level arteries and veins. Getting an accurate read-out of activity from region to region across cortical layers can help scientists understand human brain function in greater detail in health and disease.
Having shown that the technology works as expected during relatively mundane hand movements, Wang and his team are now developing the approach for fine-grained 3D mapping of brain activity throughout the many layers of the brain. This type of analysis, known as mesoscale mapping, is key to understanding dynamic activities of neural circuits that connect brain cells across cortical layers and among brain regions.
Decoding circuits, and ultimately rewiring them, is a major goal of NIH’s BRAIN Initiative. Zoomed 7T perfusion fMRI gives us a window into 4D biology, which is the ability to watch 3D objects over time scales in which life happens, whether it’s playing an elaborate drum roll or just tapping your fingers.
References:
[1] Neuroanatomical localization of the ‘precentral knob’ with computed tomography imaging. Park MC, Goldman MA, Park MJ, Friehs GM. Stereotact Funct Neurosurg. 2007;85(4):158-61.
[2]. Laminar perfusion imaging with zoomed arterial spin labeling at 7 Tesla. Shao X, Guo F, Shou Q, Wang K, Jann K, Yan L, Toga AW, Zhang P, Wang D.J.J bioRxiv 2021.04.13.439689.
Links:
Brain Basics: Know Your Brain (National Institute of Neurological Disorders and Stroke)
Laboratory of Functional MRI Technology (University of Southern California Mark and Mary Stevens Neuroimaging and Informatics Institute)
The Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your BRAINs! Photo and Video Contest (BRAIN Initiative)
NIH Support: National Institute of Neurological Disorders and Stroke; National Institute of Biomedical Imaging and Bioengineering; Office of the Director
Celebrating the Fourth with Neuroscience Fireworks
Posted on by Dr. Francis Collins
There’s so much to celebrate about our country this Fourth of July. That includes giving thanks to all those healthcare providers who have put themselves in harm’s way to staff the ERs, hospital wards, and ICUs to care for those afflicted with COVID-19, and also for everyone who worked so diligently to develop, test, and distribute COVID-19 vaccines.
These “shots of hope,” created with rigorous science and in record time, are making it possible for a great many Americans to gather safely once again with family and friends. So, if you’re vaccinated (and I really hope you are—because these vaccines have been proven safe and highly effective), fire up the grill, crank up the music, and get ready to show your true red, white, and blue colors. My wife and I—both fully vaccinated—intend to do just that!
To help get the celebration rolling, I’d like to share a couple minutes of some pretty amazing biological fireworks. While the track of a John Philip Sousa march is added just for fun, what you see in the video above is the result of some very serious neuroscience research that is scientifically, as well as visually, breath taking. Credit for this work goes to an NIH-supported team that includes Ricardo Azevedo and Sunil Gandhi, at the Center for the Neurobiology of Learning and Memory, University of California, Irvine, and their collaborator Damian Wheeler, Translucence Biosystems, Irvine, CA. Azevedo is also an NIH National Research Service Award fellow and a Medical Scientist Training Program trainee with Gandhi.
The team’s video starts off with 3D, colorized renderings of a mouse brain at cellular resolution. About 25 seconds in, the video flashes to a bundle of nerve fibers called the fornix. Thanks to the wonders of fluorescent labeling combined with “tissue-clearing” and other innovative technologies, you can clearly see the round cell bodies of individual neurons, along with the long, arm-like axons that they use to send out signals and connect with other neurons to form signaling circuits. The human brain has nearly 100 trillion of these circuits and, when activated, they process incoming sensory information and provide outputs that lead to our thoughts, words, feelings, and actions.
As shown in the video, the nerve fibers of the fornix provide a major output pathway from the hippocampus, a region of the brain involved in memory. Next, we travel to the brain’s neocortex, the outermost part of the brain that’s responsible for complex behaviors, and then move on to explore an intricate structure called the corticospinal tract, which carries motor commands to the spinal cord. The final stop is the olfactory tubercle —towards the base of the frontal lobe—a key player in odor processing and motivated behaviors.
Azevedo and his colleagues imaged the brain in this video in about 40 minutes using their imaging platform called the Translucence Biosystems’ Mesoscale Imaging System™. This process starts with a tissue-clearing method that eliminates light-scattering lipids, leaving the mouse brain transparent. From there, advanced light-sheet microscopy makes thin optical sections of the tissue, and 3D data processing algorithms reconstruct the image to high resolution.
Using this platform, researchers can take brain-wide snapshots of neuronal activity linked to a specific behavior. They can also use it to trace neural circuits that span various regions of the brain, allowing them to form new hypotheses about the brain’s connectivity and how such connectivity contributes to memory and behavior.
The video that you see here is a special, extended version of the team’s first-place video from the NIH-supported BRAIN Initiative’s 2020 “Show Us Your BRAINS!” imaging contest. Because of the great potential of this next-generation technology, Translucence Biosystems has received Small Business Innovation Research grants from NIH’s National Institute of Mental Health to disseminate its “brain-clearing” imaging technology to the neuroscience community.
As more researchers try out this innovative approach, one can only imagine how much more data will be generated to enhance our understanding of how the brain functions in health and disease. That is what will be truly spectacular for everyone working on new and better ways to help people suffering from Alzheimer’s disease, Parkinson’s disease, schizophrenia, autism, epilepsy, traumatic brain injury, depression, and so many other neurological and psychiatric disorders.
Wishing all of you a happy and healthy July Fourth!
Links:
Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
NIH National Research Service Award
Medical Scientist Training Program (National Institute of General Medical Sciences/NIH)
Small Business Innovation Research and Small Business Technology Transfer (NIH)
Translucence Biosystems (Irvine, CA)
Sunil Gandhi (University of California, Irvine)
Ricardo Azevedo (University of California, Irvine)
Video: iDISCO-cleared whole brain from a Thy1-GFP mouse (Translucence Biosystems)
Show Us Your BRAINs! Photo & Video Contest (Brain Initiative/NIH)
NIH Support: National Institute of Mental Health; National Eye Institute
Mammalian Brain Like You’ve Never Seen It Before
Posted on by Dr. Francis Collins
Credit: Gao et. al, Science
Researchers are making amazing progress in developing new imaging approaches. And they are now using one of their latest creations, called ExLLSM, to provide us with jaw-dropping views of a wide range of biological systems, including the incredibly complex neural networks within the mammalian brain.
In this video, ExLLSM takes us on a super-resolution, 3D voyage through a tiny sample (0.0030 inches thick) from the part of the mouse brain that processes sensation, the primary somatosensory cortex. The video zooms in and out of densely packed pyramidal neurons (large yellow cell bodies), each of which has about 7,000 synapses, or connections. You can also see presynapses (cyan), the part of the neuron that sends chemical signals; and postsynapes (magenta), the part of the neuron that receives chemical signals.
At 1:45, the video zooms in on dendritic spines, which are mushroom-like nubs on the neuronal branches (yellow). These structures, located on the tips of dendrites, receive incoming signals that are turned into electrical impulses. While dendritic spines have been imaged in black and white with electron microscopy, they’ve never been presented before on such a vast, colorful scale.
The video comes from a paper, published recently in the journal Science [1], from the labs of Ed Boyden, Massachusetts Institute of Technology, Cambridge, and the Nobel Prize-winning Eric Betzig, Janelia Research Campus of the Howard Hughes Medical Institute, Ashburn, VA. Like many collaborations, this one comes with a little story.
Four years ago, the Boyden lab developed expansion microscopy (ExM). The technique involves infusing cells with a hydrogel, made from a chemical used in disposable diapers. The hydrogel expands molecules within the cell away from each other, usually by about 4.5 times, but still locks them into place for remarkable imaging clarity. It makes structures visible by light microscopy that are normally below the resolution limit.
Though the expansion technique has worked well with a small number of cells under a standard light microscope, it hasn’t been as successful—until now—at imaging thicker tissue samples. That’s because thicker tissue is harder to illuminate, and flooding the specimen with light often bleaches out the fluorescent markers that scientists use to label proteins. The signal just fades away.
For Boyden, that was a problem that needed to be solved. Because his lab’s goal is to trace the inner workings of the brain in unprecedented detail, Boyden wants to image entire neural circuits in relatively thick swaths of tissue, not just look at individual cells in isolation.
After some discussion, Boyden’s team concluded that the best solution might be to swap out the light source for the standard microscope with a relatively new imaging tool developed in the Betzig lab. It’s called lattice light-sheet microscopy (LLSM), and the tool generates extremely thin sheets of light that illuminate tissue only in a very tightly defined plane, dramatically reducing light-related bleaching of fluorescent markers in the tissue sample. This allows LLSM to extend its range of image acquisition and quickly deliver stunningly vivid pictures.
Telephone calls were made, and the Betzig lab soon welcomed Ruixuan Gao, Shoh Asano, and colleagues from the Boyden lab to try their hand at combining the two techniques. As the video above shows, ExLLSM has proved to be a perfect technological match. In addition to the movie above, the team has used ExLLSM to provide unprecedented views of a range of samples—from human kidney to neuron bundles in the brain of the fruit fly.
Not only is ExLLSM super-resolution, it’s also super-fast. In fact, the team imaged the entire fruit fly brain in 2 1/2 days—an effort that would take years using an electron microscope.
ExLLSM will likely never supplant the power of electron microscopy or standard fluorescent light microscopy. Still, this new combo imaging approach shows much promise as a complementary tool for biological exploration. The more innovative imaging approaches that researchers have in their toolbox, the better for our ongoing efforts to unlock the mysteries of the brain and other complex biological systems. And yes, those systems are all complex. This is life we’re talking about!
Reference:
[1] Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Gao R, Asano SM, Upadhyayula S, Pisarev I, Milkie DE, Liu TL, Singh V, Graves A, Huynh GH, Zhao Y, Bogovic J, Colonell J, Ott CM, Zugates C, Tappan S, Rodriguez A, Mosaliganti KR, Sheu SH, Pasolli HA, Pang S, Xu CS, Megason SG, Hess H, Lippincott-Schwartz J, Hantman A, Rubin GM, Kirchhausen T, Saalfeld S, Aso Y, Boyden ES, Betzig E. Science. 2019 Jan 18;363(6424).
Links:
Video: Expansion Microscopy Explained (YouTube)
Video: Lattice Light-Sheet Microscopy (YouTube)
How to Rapidly Image Entire Brains at Nanoscale Resolution, Howard Hughes Medical Institute, January 17, 2019.
Synthetic Neurobiology Group (Massachusetts Institute of Technology, Cambridge)
Eric Betzig (Janelia Reseach Campus, Ashburn, VA)
NIH Support: National Institute of Neurological Disorders and Stroke; National Human Genome Research Institute; National Institute on Drug Abuse; National Institute of Mental Health; National Institute of Biomedical Imaging and Bioengineering
Creative Minds: Reprogramming the Brain
Posted on by Dr. Francis Collins
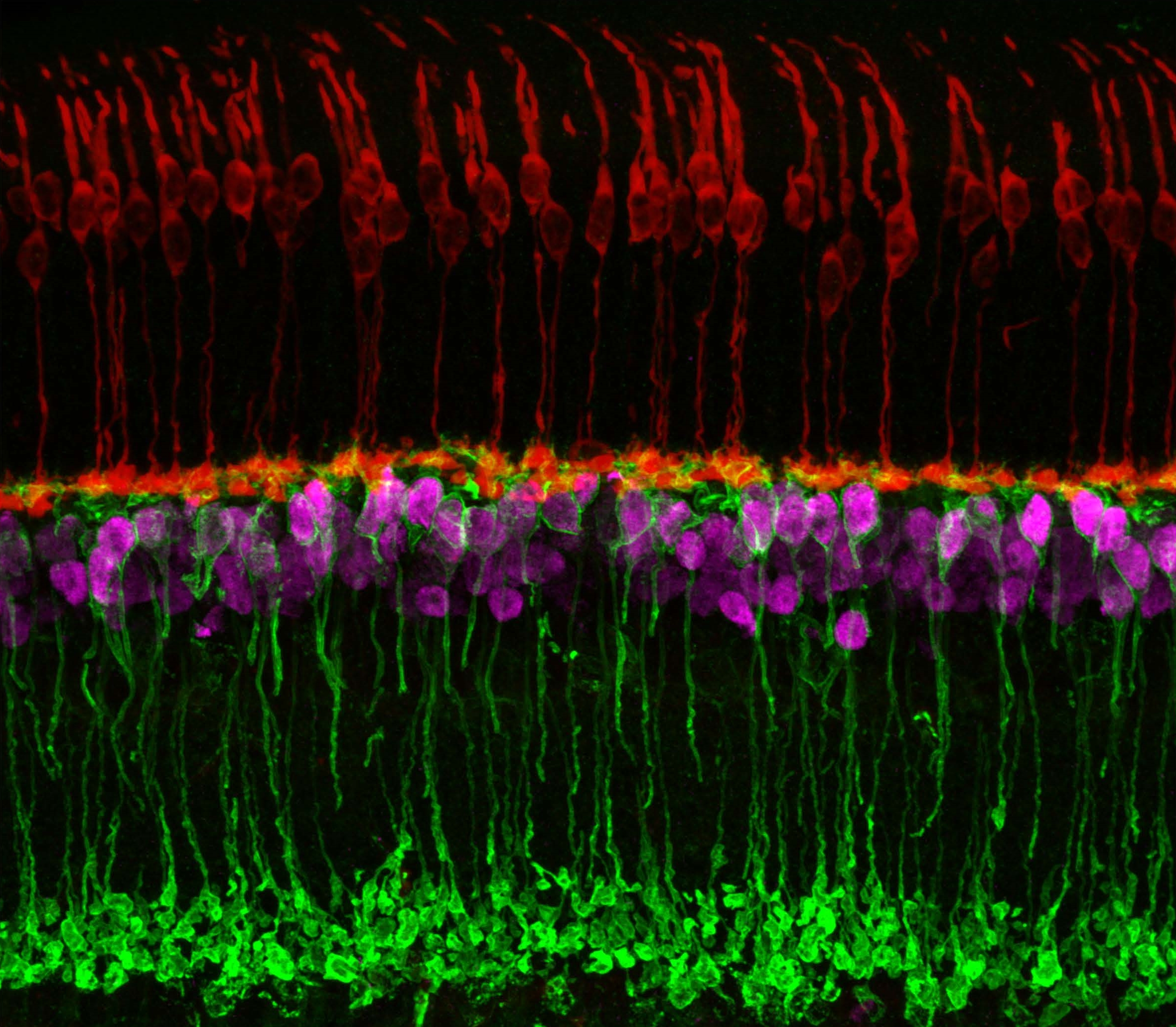
Caption: Neuronal circuits in the mouse retina. Cone photoreceptors (red) enable color vision; bipolar neurons (magenta) relay information further along the circuit; and a subtype of bipolar neuron (green) helps process signals sensed by other photoreceptors in dim light.
Credit: Brian Liu and Melanie Samuel, Baylor College of Medicine, Houston.
When most people think of reprogramming something, they probably think of writing code for a computer or typing commands into their smartphone. Melanie Samuel thinks of brain circuits, the networks of interconnected neurons that allow different parts of the brain to work together in processing information.
Samuel, a researcher at Baylor College of Medicine, Houston, wants to learn to reprogram the connections, or synapses, of brain circuits that function less well in aging and disease and limit our memory and ability to learn. She has received a 2016 NIH Director’s New Innovator Award to decipher the molecular cues that encourage the repair of damaged synapses or enable neurons to form new connections with other neurons. Because extensive synapse loss is central to most degenerative brain diseases, Samuel’s reprogramming efforts could help point the way to preventing or correcting wiring defects before they advance to serious and potentially irreversible cognitive problems.
How Sleep Resets the Brain
Posted on by Dr. Francis Collins

Caption: Colorized 3D reconstruction of dendrites. Neurons receive input from other neurons through synapses, most of which are located along the dendrites on tiny projections called spines.
Credit: The Center for Sleep and Consciousness, University of Wisconsin-Madison School of Medicine
People spend about a third of their lives asleep. When we get too little shut-eye, it takes a toll on attention, learning and memory, not to mention our physical health. Virtually all animals with complex brains seem to have this same need for sleep. But exactly what is it about sleep that’s so essential?
Two NIH-funded studies in mice now offer a possible answer. The two research teams used entirely different approaches to reach the same conclusion: the brain’s neural connections grow stronger during waking hours, but scale back during snooze time. This sleep-related phenomenon apparently keeps neural circuits from overloading, ensuring that mice (and, quite likely humans) awaken with brains that are refreshed and ready to tackle new challenges.
