asthma
Predicting ‘Long COVID Syndrome’ with Help of a Smartphone App
Posted on by Dr. Francis Collins

As devastating as this pandemic has been, it’s truly inspiring to see the many innovative ways in which researchers around the world have enlisted the help of everyday citizens to beat COVID-19. An intriguing example is the COVID Symptom Study’s smartphone-based app, which already has been downloaded millions of times, mostly in the United States and United Kingdom. Analyzing data from 2.6 million app users, researchers published a paper last summer showing that self-reported symptoms can help to predict infection with SARS-CoV-2, the coronavirus that causes COVID-19 [1].
New work from the COVID Symptom Study now takes advantage of the smartphone app to shed more light on Long COVID Syndrome [2], in which people experience a constellation of symptoms long past the time that they’ve recovered from the initial stages of COVID-19 illness. Such symptoms, which can include fatigue, shortness of breath, “brain fog,” sleep disorders, fevers, gastrointestinal symptoms, anxiety, and depression, can persist for months and can range from mild to incapacitating
This latest findings, published in the journal Nature Medicine, come from a team led by Claire Steves and Tim Spector, King’s College London, and their colleagues, and that includes NIH grantee Andrew Chan, Massachusetts General Hospital, Boston, and others supported by the Massachusetts Consortium on Pathogen Readiness. The team began by looking at data recorded between March 24-Sept. 2, 2020 from about 4.2 million app users with an average age of 45, about 90 percent of whom lived in the U.K., with smaller numbers from the U.S. and Sweden.
For this particular study, the researchers decided to focused on 4,182 app users, all with confirmed COVID-19, who had consistently logged in their symptoms. Because these individuals also started using the app when they still felt physically well, the researchers could assess their COVID-19-associated symptoms over the course of the illness.
While most people who developed COVID-19 were back to normal in less than two weeks, the data suggest that one in 20 people with COVID-19 are likely to suffer symptoms of Long COVID that persist for eight weeks or more. About one in 50 people continued to have symptoms for 12 weeks or more. That suggests Long COVID could potentially affect many hundreds of thousands of people in the U.K. alone and millions more worldwide.
The team found that the individuals most likely to develop Long COVID were older people, women, and especially those who experienced five or more symptoms. The nature and order of symptoms, which included fatigue, headache, shortness of breath, and loss of smell, didn’t matter. People with asthma also were more likely to develop long-lasting symptoms, although the study found no clear links to any other pre-existing health conditions.
Using this information, the researchers developed a model to predict which individuals were most likely to develop Long COVID. Remarkably, this simple algorithm—based on age, gender, and number of early symptoms–accurately predicted almost 70 percent of cases of Long COVID. It was also about 70 percent effective in avoiding false alarms.
The team also validated the algorithm’s predictive ability in data from an independent group of 2,472 people with confirmed COVID-19 and a range of symptoms. In this group, having more than five symptoms within the first week also proved to be the strongest predictor of Long COVID. And, again, the model worked quite well in identifying those most likely to develop Long COVID.
These findings come as yet another important reminder of the profound impact of the COVID-19 pandemic on public health. This includes not only people who are hospitalized with severe COVID-19 but, all too often, those who get through the initial period of infection relatively unscathed.
Recently, NIH announced a $1.15 billion investment to identify the causes of Long COVID, to develop ways of treating individuals who don’t fully recover, and, ultimately, to prevent the disorder. We’ve been working diligently in recent weeks to identify the most pressing questions and areas of greatest opportunity to address this growing public health threat. As a first step, NIH is funding an effort to track the recovery paths of at least 40,000 adults and children infected with SARS-CoV-2, to learn more about who develops long-term effects and who doesn’t. If you’d like to find a way to pitch in and help, getting involved in the COVID Symptom Study is as easy as downloading the app.
References:
[1] Real-time tracking of self-reported symptoms to predict potential COVID-19. Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, Ganesh S, Varsavsky T, Cardoso MJ, El-Sayed Moustafa JS, Visconti A, Hysi P, Bowyer RCE, Mangino M, Falchi M, Wolf J, Ourselin S, Chan AT, Steves CJ, Spector TD. Nat Med. 2020 Jul;26(7):1037-1040. doi: 10.1038/s41591-020-0916-2. Epub 2020 May 11.
[2] Attributes and predictors of long COVID. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ. Nat Med. 2021 Mar 10.
Links:
NIH launches new initiative to study to “Long COVID”. 2021 Feb 23. (NIH)
COVID-19 Research (NIH)
Massachusetts Consortium on Pathogen Readiness (Boston)
Claire Steves (King’s College London, United Kingdom)
Tim Spector (King’s College London)
Andrew Chan (Massachusetts General Hospital, Boston)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
These Oddball Cells May Explain How Influenza Leads to Asthma
Posted on by Dr. Francis Collins
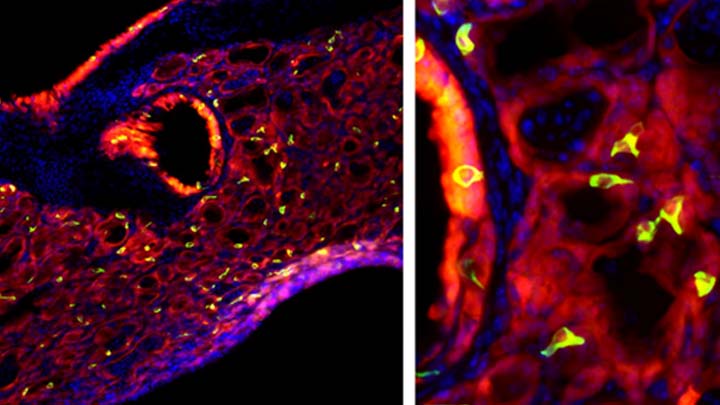
Most people who get the flu bounce right back in a week or two. But, for others, the respiratory infection is the beginning of lasting asthma-like symptoms. Though I had a flu shot, I had a pretty bad respiratory illness last fall, and since that time I’ve had exercise-induced asthma that has occasionally required an inhaler for treatment. What’s going on? An NIH-funded team now has evidence from mouse studies that such long-term consequences stem in part from a surprising source: previously unknown lung cells closely resembling those found in taste buds.
The image above shows the lungs of a mouse after a severe case of H1N1 influenza infection, a common type of seasonal flu. Notice the oddball cells (green) known as solitary chemosensory cells (SCCs). Those little-known cells display the very same chemical-sensing surface proteins found on the tongue, where they allow us to sense bitterness. What makes these images so interesting is, prior to infection, the healthy mouse lungs had no SCCs.
SCCs, sometimes called “tuft cells” or “brush cells” or “type II taste receptor cells”, were first described in the 1920s when a scientist noticed unusual looking cells in the intestinal lining [1] Over the years, such cells turned up in the epithelial linings of many parts of the body, including the pancreas, gallbladder, and nasal passages. Only much more recently did scientists realize that those cells were all essentially the same cell type. Owing to their sensory abilities, these epithelial cells act as a kind of lookout for signs of infection or injury.
This latest work on SCCs, published recently in the American Journal of Physiology–Lung Cellular and Molecular Physiology, adds to this understanding. It comes from a research team led by Andrew Vaughan, University of Pennsylvania School of Veterinary Medicine, Philadelphia [2].
As a post-doc, Vaughan and colleagues had discovered a new class of cells, called lineage-negative epithelial progenitors, that are involved in abnormal remodeling and regrowth of lung tissue after a serious respiratory infection [3]. Upon closer inspection, they noticed that the remodeling of lung tissue post-infection often was accompanied by sustained inflammation. What they didn’t know was why.
The team, including Noam Cohen of Penn’s Perelman School of Medicine and De’Broski Herbert, also of Penn Vet, noticed signs of an inflammatory immune response several weeks after an influenza infection. Such a response in other parts of the body is often associated with allergies and asthma. All were known to involve SCCs, and this begged the question: were SCCs also present in the lungs?
Further work showed not only were SCCs present in the lungs post-infection, they were interspersed across the tissue lining. When the researchers exposed the animals’ lungs to bitter compounds, the activated SCCs multiplied and triggered acute inflammation.
Vaughan’s team also found out something pretty cool. The SCCs arise from the very same lineage of epithelial progenitor cells that Vaughan had discovered as a post-doc. These progenitor cells produce cells involved in remodeling and repair of lung tissue after a serious lung infection.
Of course, mice aren’t people. The researchers now plan to look in human lung samples to confirm the presence of these cells following respiratory infections.
If confirmed, the new findings might help to explain why kids who acquire severe respiratory infections early in life are at greater risk of developing asthma. They suggest that treatments designed to control these SCCs might help to treat or perhaps even prevent lifelong respiratory problems. The hope is that ultimately it will help to keep more people breathing easier after a severe bout with the flu.
References:
[1] Closing in on a century-old mystery, scientists are figuring out what the body’s ‘tuft cells’ do. Leslie M. Science. 2019 Mar 28.
[2] Development of solitary chemosensory cells in the distal lung after severe influenza injury. Rane CK, Jackson SR, Pastore CF, Zhao G, Weiner AI, Patel NN, Herbert DR, Cohen NA, Vaughan AE. Am J Physiol Lung Cell Mol Physiol. 2019 Mar 25.
[3] Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Nature. 2015 Jan 29;517(7536):621-625.
Links:
Asthma (National Heart, Lung, and Blood Institute/NIH)
Influenza (National Institute of Allergy and Infectious Diseases/NIH)
Vaughan Lab (University of Pennsylvania, Philadelphia)
Cohen Lab (University of Pennsylvania, Philadelphia)
Herbert Lab (University of Pennsylvania, Philadelphia)
NIH Support: National Heart, Lung, and Blood Institute; National Institute on Deafness and Other Communication Disorders
Are E-cigarettes Leading More Kids to Smoke?
Posted on by Dr. Francis Collins

Thinkstock\MilknCoffee
Today, thanks to decades of educational efforts about the serious health consequences of inhaled tobacco, fewer young people than ever smoke cigarettes in the United States. So, it’s interesting that a growing of number of middle and high school kids are using e-cigarettes—electronic devices that vaporize flavored liquid that generally contains nicotine.
E-cigarettes come with their own health risks, including lung inflammation, asthma, and respiratory infections. But their supporters argue that “vaping,” as it’s often called, might provide an option that would help young people steer clear of traditional cigarettes and the attendant future risks of lung cancer, emphysema, heart disease, and other serious health conditions. Now, a new NIH-funded study finds that this is—pardon the pun—mostly a pipe dream.
Analyzing the self-reported smoking behaviors of thousands of schoolkids nationwide, researchers found no evidence that the availability of e-cigarettes has served to accelerate the decline in youth smoking. In fact, the researchers concluded the opposite: the popularity of e-cigarettes has led more kids—not fewer—to get hooked on nicotine, which meets all criteria for being an addictive substance.
Imaging Advance Offers New View on Allergic Asthma
Posted on by Dr. Francis Collins

Caption: OR-OCT images of the airways of a healthy person (left) and a person with allergic asthma (right). The colorized portion highlights airway smooth muscle, with thinner areas in purple and black and thicker areas in yellow and orange. Credit: Cho et al., Science Translational Medicine (2016)
You probably know people who sneeze a little when they encounter plant pollens, pet dander, or other everyday allergens. For others, however, these same allergens can trigger a serious asthma attack that can make breathing a life-or-death struggle. Now, two NIH-funded research groups have teamed up to help explain the differences in severity underlying the two types of reactions.
In the studies, researchers at Massachusetts General Hospital, Boston, used an innovative imaging tool to zoom in on a person’s airways safely in real time to gain an unprecedented view of how his or her body reacts to allergens [1,2]. The imaging revealed key differences between the asthma and non-asthma groups in the smooth muscle tissue that surrounds critical airways, and is responsible for constriction. In a complementary series of experiments, researchers also uncovered heightened immune responses in the airways of folks with allergic asthma. The findings offer important new clues in the quest to better understand and guide treatment for asthma, a condition that affects more than 300 million people around the world.
The factors driving airway constriction in people with asthma have been poorly understood in part because, until now, there hasn’t been a way to view airway smooth muscle in action. As described in the journal Science Translational Medicine, Melissa Suter and colleagues adapted an established form of imaging called optical coherence tomography (OCT) to help fill this gap. Standard OCT produces an image by measuring the amount of light reflected back from body tissues, but such images aren’t sufficient to distinguish airway smooth muscle from other tissues.
Largest Study Yet Shows Mother’s Smoking Changes Baby’s Epigenome
Posted on by Dr. Francis Collins

Credit: Daniel Berehulak/Getty Images
Despite years of public health campaigns warning of the dangers of smoking when pregnant, many women are unaware of the risk or find themselves unable to quit. As a result, far too many babies are still being exposed in the womb to toxins that enter their mothers’ bloodstreams when they inhale cigarette smoke. Among the many infant and child health problems that have been linked to maternal smoking are premature birth, low birth weight, asthma, reduced lung function, sudden infant death syndrome (SIDS), and cleft lip and/or palate.
Now, a large international study involving NIH-supported researchers provides a biological mechanism that may explain how exposure to cigarette toxins during fetal development can produce these health problems [1]. That evidence centers on the impact of the toxins on the epigenome of the infant’s body tissues. The epigenome refers to chemical modifications of DNA (particularly methylation of cytosines), as well as proteins that bind to DNA and affect its function. The genome of an individual is the same in all cells of their body, but the epigenome determines whether genes are turned on or off in particular cells. The study found significant differences between the epigenetic patterns of babies born to women who smoked during pregnancy and those born to non-smokers, with many of the differences affecting genes known to play key roles in the development of the lungs, face, and nervous system.
Creative Minds: Harnessing Technologies to Study Air Pollution’s Health Risks
Posted on by Dr. Francis Collins
After college, Perry Hystad took a trip to India and, while touring several large cities, noticed the vast clouds of exhaust from vehicles, smoke from factories, and soot from biomass-burning cook stoves. As he watched the rapid urban expansion all around him, Hystad remembers thinking: What effect does breathing such pollution day in and day out have upon these people’s health?
This question stuck with Hystad, and he soon developed a profound interest in environmental health. In 2013, Hystad completed his Ph.D. in his native Canada, studying the environmental risk factors for lung cancer [1, 2, 3]. Now, with the support of an NIH Director’s Early Independence Award, Hystad has launched his own lab at Oregon State University, Corvallis, to investigate further the health impacts of air pollution, which one recent analysis indicates may contribute to as many as several million deaths worldwide each year [4].
Tackling Health Disparities: Childhood Asthma
Posted on by Dr. Francis Collins
One condition for which NIH researchers are working to reduce disparities is asthma, the most common chronic condition that keeps kids home from school.
Compared to white children, Puerto Rican youngsters are 2.4 times more likely to suffer from asthma, African Americans, 1.6 times; and American Indians/Alaska Natives, 1.3 times.
Source: National Heart, Lung, and Blood Institute, NIH



