immune
Mapping Immune Cell “Neighborhoods” in Psoriasis to Understand its Course
Posted on by Lawrence Tabak, D.D.S., Ph.D.

“Location, location, location.” While most of us know this phrase as a real estate adage, location—specifically that of various cell types—is becoming a key area of investigation in studying human disease. New techniques are enabling scientists to understand where certain cells are with respect to one another and how changes in their activity may affect your overall health.
In one recent example of the power of this approach, NIH-funded researchers [1] used a sophisticated method to map immune cells within human skin to get a more detailed picture of psoriasis, a common, chronic disease in which the immune system becomes overactive leading to skin inflammation. People with psoriasis develop patches of itchy, red, and flaky lesions on their skin, which can be mild to severe. For reasons that aren’t entirely clear, they’re also at higher risk for developing a wide range of other health conditions, including a unique form of arthritis known as psoriatic arthritis, diabetes, mental health issues, heart problems, and more.
The hope is that these newly drawn, precise maps of cellular “neighborhoods” in human skin will help chart the precise course of this disease to understand better the differences between mild and more severe forms. They may also yield important clues as to why people with psoriasis develop other health problems more often than people without psoriasis.
In the new study, a team including Jose Scher and Shruti Naik, NYU Langone, New York, analyzed immune cells within 25 skin samples from 14 volunteers, including those with active psoriasis, those with psoriasis but no active lesions, and people with healthy skin who do not have psoriasis. The researchers relied on a sophisticated approach called spatial transcriptomics [2] to map out what happens at the single-cell level within the samples.
In earlier approaches to single-cell analysis, researchers first would separate cells from the tissue they came from. While they could measure gene activity within those cells at the individual level, they couldn’t put things back together to see how they all fit. With spatial transcriptomics, it’s now possible to molecularly profile single cells to measure their activity in a tissue sample while also mapping their locations with respect to other cells.
The new study led to some intriguing findings. For instance, certain immune cells, specifically B cells, moved to the upper layers of the skin during active disease. That’s notable because prior studies had been unable to capture B cells in the skin adequately, and these cells are thought to play an important role in the disease.
Interestingly, the spatial cellular maps revealed inflammatory regions in both actively inflamed skin and in skin that appeared healthy. This finding highlights the fact that the inflammation that goes with psoriasis can affect the skin, and likely other parts of the body, in ways that aren’t easily observed. In future studies, the researchers want to explore how the presence of psoriasis and its underlying changes in immune cell activity may influence other organs and tissues beneath the skin.
Their fine-scale maps also showed increased gene activity in dozens of molecular pathways that are tied to metabolism and the control of lipid levels. That’s especially interesting because these factors are known to go awry in diabetes and heart conditions, which happen more often in people with psoriasis compared to those without. They also could see in their maps that this altered activity sometimes occurred in clear skin distant from any apparent lesions.
Having discovered such signals with potential consequences for other parts of the body, the researchers report that they’re working to understand how inflammatory immune cells and processes in the skin may lead to more widespread disease processes that affect other parts of the body. They plan to conduct similar studies in larger groups of people with and without active psoriasis lesions and studies following individuals with psoriasis over time. They’ll also explore questions about why people respond differently to the same anti-inflammatory treatment regimens.
To speed the process of discovery, they’ve made their maps and associated data freely available as a resource for the scientific community. About 7.5 million adults in the U.S. and millions more worldwide have psoriasis and associated psoriatic conditions [3]. The hope is that these maps will one day help to steer them toward a healthier future.
References:
[1] Spatial transcriptomics stratifies psoriatic disease severity by emergent cellular ecosystems. Castillo RL, Sidhu I, Dolgalev I, Chu T, Prystupa A, Subudhi I, Yan D, Konieczny P, Hsieh B, Haberman RH, Selvaraj S, Shiomi T, Medina R, Girija PV, Heguy A, Loomis CA, Chiriboga L, Ritchlin C, Garcia-Hernandez ML, Carucci J, Meehan SA, Neimann AL, Gudjonsson JE, Scher JU, Naik S. Sci Immunol. 2023 Jun 8;8(84):eabq7991. doi: 10.1126/sciimmunol.abq7991.
[2] Method of the Year: spatially resolved transcriptomics. Marx V. Nat Methods. 2021 Jan;18(1):9-14. doi: 10.1038/s41592-020-01033-y.
[3] Psoriasis Prevalence in Adults in the United States. Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. JAMA Dermatol. 2021 Aug 1;157(8):940-946. doi: 10.1001/jamadermatol.2021.2007.
Links:
Psoriasis (National Institute of Arthritis and Musculoskeletal and Skin Diseases/NIH)
Jose Scher (NYU Langone Health, New York, NY)
Shruti Naik (NYU Langone Health, New York, NY)
NIH Support: National Cancer Institute, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Center for Advancing Translational Sciences, National Institute of Allergy and Infectious Diseases
Imaging Advance Offers New View on Allergic Asthma
Posted on by Dr. Francis Collins

Caption: OR-OCT images of the airways of a healthy person (left) and a person with allergic asthma (right). The colorized portion highlights airway smooth muscle, with thinner areas in purple and black and thicker areas in yellow and orange. Credit: Cho et al., Science Translational Medicine (2016)
You probably know people who sneeze a little when they encounter plant pollens, pet dander, or other everyday allergens. For others, however, these same allergens can trigger a serious asthma attack that can make breathing a life-or-death struggle. Now, two NIH-funded research groups have teamed up to help explain the differences in severity underlying the two types of reactions.
In the studies, researchers at Massachusetts General Hospital, Boston, used an innovative imaging tool to zoom in on a person’s airways safely in real time to gain an unprecedented view of how his or her body reacts to allergens [1,2]. The imaging revealed key differences between the asthma and non-asthma groups in the smooth muscle tissue that surrounds critical airways, and is responsible for constriction. In a complementary series of experiments, researchers also uncovered heightened immune responses in the airways of folks with allergic asthma. The findings offer important new clues in the quest to better understand and guide treatment for asthma, a condition that affects more than 300 million people around the world.
The factors driving airway constriction in people with asthma have been poorly understood in part because, until now, there hasn’t been a way to view airway smooth muscle in action. As described in the journal Science Translational Medicine, Melissa Suter and colleagues adapted an established form of imaging called optical coherence tomography (OCT) to help fill this gap. Standard OCT produces an image by measuring the amount of light reflected back from body tissues, but such images aren’t sufficient to distinguish airway smooth muscle from other tissues.
Snapshots of Life: NIH’s BioArt Winners
Posted on by Dr. Francis Collins
If you follow my blog, you know that I like to feature spectacular images that scientists have created during the course of their research. These images are rarely viewed outside the lab, but some are so worthy of artistic merit and brimming with educational value that they deserve a wider audience. That’s one reason why the Federation of American Societies for Experimental Biology (FASEB) launched its BioArt contest. Of the 12 winners in 2013, I’m proud to report that 11 received support from NIH. In fact, I’m so proud that I plan to showcase their work in an occasional series entitled “Snapshots of Life.” Continue reading to see the first installment—enjoy!
‘No Ouch’ Vaccine Patch
Posted on by Dr. Francis Collins

A vaccine patch and a view of the “needles” using scanning electron microscopy.
Credit: Peter DeMuth/Wellcome Trust
This might be a new way to get a shot. Funded in part by the NIH, this vaccine patch [1] is coated in a thin film that literally melts into the skin when the patch is applied. The film contains DNA, rather than protein, which is absorbed by the skin cells and triggers an immune reaction. It seems to be effective in animal models. DNA vaccines are attractive because they may not require refrigeration like typical protein vaccines and can be stably stored for weeks. And, though this patch looks spiky, the length of the needles can be adjusted so that they don’t reach the skin layers that contain nerves. Thus: no ouch.
[1] Polymer multilayer tattooing for enhanced DNA vaccination. Demuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, Hammond PT, Irvine DJ. Nat Mater. 2013 Jan 27.
NIH support: the National Institute of Allergy and Infectious Diseases
Taking a Snapshot of the Human Immune System
Posted on by Dr. Francis Collins
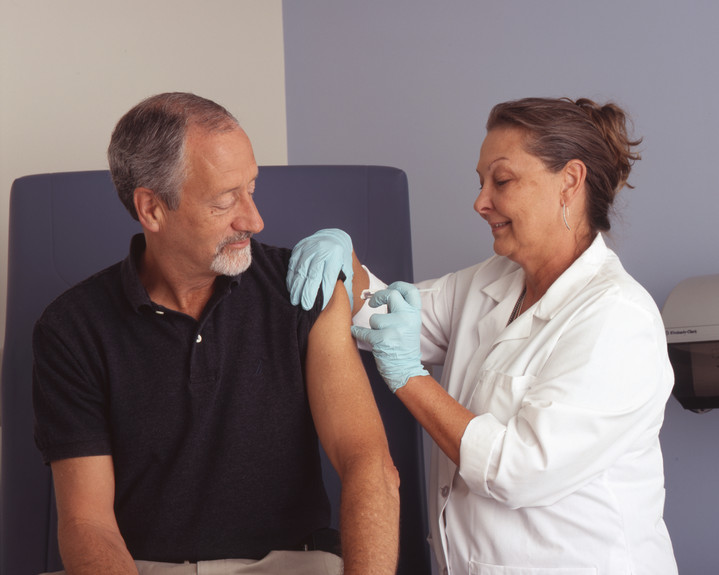
Source: National Cancer Institute Visuals Online, NIH
There are numerous tests to gauge the health of your heart. But no such widely accepted test exists for the many parts of the immune system. How can we tell if the immune system is strong or weak? Or quantify how badly it’s malfunctioning when we suffer from asthma, allergies, or arthritis?
A team led by scientists at Stanford University has taken the first steps toward creating such a test—by taking “snapshots” of the immune system.
Before we talk about what they did, let me review how the immune system protects us against disease. The innate immune system is like a standing army that defends us against invading microbes. But the innate system has no memory. It doesn’t recognize the invaders more quickly if they return. This is the job of the adaptive immune system—B and T cells. These cells not only remember invaders; they’re able to adapt their weapons—antibodies and T-cell receptors—to make them more effective. Think of them as the Special Forces.
Who Knew? Gut Bacteria Contribute to Malnutrition
Posted on by Dr. Francis Collins

A child suffering from kwashiorkor.
Source: CDC/Phil
Here’s a surprising result from a new NIH-funded study: a poor diet isn’t the only cause of severe malnutrition. It seems that a ‘bad’ assortment of microbes in the intestine can conspire with a nutrient poor diet to promote and perpetuate malnutrition [1].
Most of us don’t spend time thinking about it, but healthy humans harbor about 100 trillion bacteria in our intestines and trillions more in our nose, mouth, skin, and urogenital tracts. And though your initial reaction might be “yuck,” the presence of these microbes is generally a good thing. We’ve evolved with this bacterial community because they provide services—from food digestion to bolstering the immune response—and we give them food and shelter. We call these bacterial sidekicks our ‘microbiome,’ and the latest research, much of it NIH-funded, reveals that these life passengers are critical for good health. You read that right—we need bacteria. The trouble starts when the wrong ones take up residence in our body, or the bacterial demographics shift. Then diseases from eczema and obesity to asthma and heart disease may result. Indeed, we’ve learned that microbes even modulate our sex hormones and influence the risk of autoimmune diseases like type 1 diabetes. [2]

