seizures
How Our Brains Replay Memories
Posted on by Dr. Francis Collins

Note to my blog readers: the whole world is now facing a major threat from the COVID-19 pandemic. We at NIH are doing everything we can to apply the best and most powerful science to the development of diagnostics, therapeutics, and vaccines, while also implementing public health measures to protect our staff and the patients in our hospital. This crisis is expected to span many weeks, and I will occasionally report on COVID-19 in this blog format. Meanwhile, science continues to progress on many other fronts—and so I will continue to try to bring you stories across a wide range of topics. Perhaps everyone can use a little break now and then from the coronavirus news? Today’s blog takes you into the intricacies of memory.
When recalling the name of an acquaintance, you might replay an earlier introduction, trying to remember the correct combination of first and last names. (Was it Scott James? Or James Scott?) Now, neuroscientists have found that in the split second before you come up with the right answer, your brain’s neurons fire in the same order as when you first learned the information [1].
This new insight into memory retrieval comes from recording the electrical activity of thousands of neurons in the brains of six people during memory tests of random word pairs, such as “jeep” and “crow.” While similar firing patterns had been described before in mice, the new study is the first to confirm that the human brain stores memories in specific sequences of neural activity that can be replayed again and again.
The new study, published in the journal Science, is the latest insight from neurosurgeon and researcher Kareem Zaghloul at NIH’s National Institute of Neurological Disorders and Stroke (NINDS). Zaghloul’s team has for years been involved in an NIH Clinical Center study for patients with drug-resistant epilepsy whose seizures cannot be controlled with drugs.
As part of this work, his surgical team often temporarily places a 4 millimeter-by-4 millimeter array of tiny electrodes on the surface of the brains of the study’s participants. They do this in an effort to pinpoint brain tissues that may be the source of their seizures before performing surgery to remove them. With a patient’s informed consent to take part in additional research, the procedure also has led to a series of insights into what happens in the human brain when we make and later retrieve new memories.
Here’s how it works: The researchers record electrical currents as participants are asked to learn random word pairs presented to them on a computer screen, such as “cake” and “fox,” or “lime” and “camel.” After a period of rest, their brain activity is again recorded as they are given a word and asked to recall the matching word.
Last year, the researchers reported that the split second before a person got the right answer, tiny ripples of electrical activity appeared in two specific areas of the brain [2]. The team also had shown that, when a person correctly recalled a word pair, the brain showed patterns of activity that corresponded to those formed when he or she first learned to make a word association.
The new work takes this a step further. As study participants learned a word pair, the researchers noticed not only the initial rippling wave of electricity, but also that particular neurons in the brain’s cerebral cortex fired repeatedly in a sequential order. In fact, with each new word pair, the researchers observed unique firing patterns among the active neurons.
If the order of neuronal firing was essential for storing new memories, the researchers reasoned that the same would be true for correctly retrieving the information. And, indeed, that’s what they were able to show. For example, when individuals were shown “cake” for a second time, they replayed a very similar firing pattern to the one recorded initially for this word just milliseconds before correctly recalling the paired word “fox.”
The researchers then calculated the average sequence similarity between the firing patterns of learning and retrieval. They found that as a person recalled a word, those patterns gradually became more similar. Just before a correct answer was given, the recorded neurons locked onto the right firing sequence. That didn’t happen when a person gave an incorrect answer.
Further analysis confirmed that the exact order of neural firing was specific to each word pair. The findings show that our memories are encoded as unique sequences that must be replayed for accurate retrieval, though we still don’t understand the molecular mechanisms that undergird this.
Zaghloul reports that there’s still more to learn about how these processes are influenced by other factors such as our attention. It’s not yet known whether the brain replays sequences similarly when retrieving longer-term memories. Along with these intriguing insights into normal learning and memory, the researchers think this line of research will yield important clues as to what changes in people who suffer from memory disorders, with potentially important implications for developing the next generation of treatments.
Reference:
[1] Replay of cortical spiking sequences during human memory retrieval. Vaz AP, Wittig JH Jr, Inati SK, Zaghloul KA. Science. 2020 Mar 6;367(6482):1131-1134.
[2] Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Vaz AP, Inati SK, Brunel N, Zaghloul KA. Science. 2019 Mar 1;363(6430):975-978.
Links:
Epilepsy Information Page (National Institute of Neurological Disorders and Stroke/NIH)
Brain Basics (NINDS)
Zaghloul Lab (NINDS)
NIH Support: National Institute of Neurological Disorders and Stroke; National Institute of General Medical Sciences
Discovering a Source of Laughter in the Brain
Posted on by Dr. Francis Collins
If laughter really is the best medicine, wouldn’t it be great if we could learn more about what goes on in the brain when we laugh? Neuroscientists recently made some major progress on this front by pinpointing a part of the brain that, when stimulated, never fails to induce smiles and laughter.
In their study conducted in three patients undergoing electrical stimulation brain mapping as part of epilepsy treatment, the NIH-funded team found that stimulation of a specific tract of neural fibers, called the cingulum bundle, triggered laughter, smiles, and a sense of calm. Not only do the findings shed new light on the biology of laughter, researchers hope they may also lead to new strategies for treating a range of conditions, including anxiety, depression, and chronic pain.
In people with epilepsy whose seizures are poorly controlled with medication, surgery to remove seizure-inducing brain tissue sometimes helps. People awaiting such surgeries must first undergo a procedure known as intracranial electroencephalography (iEEG). This involves temporarily placing 10 to 20 arrays of tiny electrodes in the brain for up to several weeks, in order to pinpoint the source of a patient’s seizures in the brain. With the patient’s permission, those electrodes can also enable physician-researchers to stimulate various regions of the patient’s brain to map their functions and make potentially new and unexpected discoveries.
In the new study, published in The Journal of Clinical Investigation, Jon T. Willie, Kelly Bijanki, and their colleagues at Emory University School of Medicine, Atlanta, looked at a 23-year-old undergoing iEEG for 8 weeks in preparation for surgery to treat her uncontrolled epilepsy [1]. One of the electrodes implanted in her brain was located within the cingulum bundle and, when that area was stimulated for research purposes, the woman experienced an uncontrollable urge to laugh. Not only was the woman given to smiles and giggles, she also reported feeling relaxed and calm.
As a further and more objective test of her mood, the researchers asked the woman to interpret the expression of faces on a computer screen as happy, sad, or neutral. Electrical stimulation to the cingulum bundle led her to see those faces as happier, a sign of a generally more positive mood. A full evaluation of her mental state also showed she was fully aware and alert.
To confirm the findings, the researchers looked to two other patients, a 40-year-old man and a 28-year-old woman, both undergoing iEEG in the course of epilepsy treatment. In those two volunteers, stimulation of the cingulum bundle also triggered laughter and reduced anxiety with otherwise normal cognition.
Willie notes that the cingulum bundle links many brain areas together. He likens it to a super highway with lots of on and off ramps. He suspects the spot they’ve uncovered lies at a key intersection, providing access to various brain networks regulating mood, emotion, and social interaction.
Previous research has shown that stimulation of other parts of the brain can also prompt patients to laugh. However, what makes stimulation of the cingulum bundle a particularly promising approach is that it not only triggers laughter, but also reduces anxiety.
The new findings suggest that stimulation of the cingulum bundle may be useful for calming patients’ anxieties during neurosurgeries in which they must remain awake. In fact, Willie’s team did so during their 23-year-old woman’s subsequent epilepsy surgery. Each time she became distressed, the stimulation provided immediate relief. Also, if traditional deep brain stimulation or less invasive means of brain stimulation can be developed and found to be safe for long-term use, they may offer new ways to treat depression, anxiety disorders, and/or chronic pain.
Meanwhile, Willie’s team is hard at work using similar approaches to map brain areas involved in other aspects of mood, including fear, sadness, and anxiety. Together with the multidisciplinary work being mounted by the NIH-led BRAIN Initiative, these kinds of studies promise to reveal functionalities of the human brain that have previously been out of reach, with profound consequences for neuroscience and human medicine.
Reference:
[1] Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy. Bijanki KR, Manns JR, Inman CS, Choi KS, Harati S, Pedersen NP, Drane DL, Waters AC, Fasano RE, Mayberg HS, Willie JT. J Clin Invest. 2018 Dec 27.
Links:
Video: Patient’s Response (Bijanki et al. The Journal of Clinical Investigation)
Epilepsy Information Page (National Institute of Neurological Disease and Stroke/NIH)
Jon T. Willie (Emory University, Atlanta, GA)
NIH Support: National Institute of Neurological Disease and Stroke; National Center for Advancing Translational Sciences
Distinctive Brain ‘Subnetwork’ Tied to Feeling Blue
Posted on by Dr. Francis Collins

Credit: :iStock/kieferpix
Experiencing a range of emotions is a normal part of human life, but much remains to be discovered about the neuroscience of mood. In a step toward unraveling some of those biological mysteries, researchers recently identified a distinctive pattern of brain activity associated with worsening mood, particularly among people who tend to be anxious.
In the new study, researchers studied 21 people who were hospitalized as part of preparation for epilepsy surgery, and took continuous recordings of the brain’s electrical activity for seven to 10 days. During that same period, the volunteers also kept track of their moods. In 13 of the participants, low mood turned out to be associated with stronger activity in a “subnetwork” that involved crosstalk between the brain’s amygdala, which mediates fear and other emotions, and the hippocampus, which aids in memory.
How the Brain Regulates Vocal Pitch
Posted on by Dr. Francis Collins
Credit: University of California, San Francisco
Whether it’s hitting a high note, delivering a punch line, or reading a bedtime story, the pitch of our voices is a vital part of human communication. Now, as part of their ongoing quest to produce a dynamic picture of neural function in real time, researchers funded by the NIH’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative have identified the part of the brain that controls vocal pitch [1].
This improved understanding of how the human brain regulates the pitch of sounds emanating from the voice box, or larynx, is more than cool neuroscience. It could aid in the development of new, more natural-sounding technologies to assist people who have speech disorders or who’ve had their larynxes removed due to injury or disease.
Rare Disease Mystery: Nodding Syndrome May Be Linked to Parasitic Worm
Posted on by Dr. Francis Collins

Caption: Village in the East Africa nation of Uganda
Credit: Centers for Disease Control and Prevention
In the early 1960s, reports began to surface that some children living in remote villages in East Africa were suffering mysterious episodes of “head nodding.” The condition, now named nodding syndrome, is recognized as a rare and devastating form of epilepsy. There were hints that the syndrome might be caused by a parasitic worm called Onchocerca volvulus, which is transmitted through the bites of blackflies. But no one had been able to tie the parasitic infection directly to the nodding heads.
Now, NIH researchers and their international colleagues think they’ve found the missing link. The human immune system turns out to be a central player. After analyzing blood and cerebrospinal fluid of kids with nodding syndrome, they detected a particular antibody at unusually high levels [1]. Further studies suggest the immune system ramps up production of that antibody to fight off the parasite. The trouble is those antibodies also react against a protein in healthy brain tissue, apparently leading to progressive cognitive dysfunction, neurological deterioration, head nodding, and potentially life-threatening seizures.
The findings, published in Science Translational Medicine, have important implications for the treatment and prevention of not only nodding syndrome, but perhaps other autoimmune-related forms of epilepsy. As people in the United States and around the globe today observe the 10th anniversary of international Rare Disease Day, this work provides yet another example of how rare disease research can shed light on more common diseases and fundamental aspects of human biology.
Brain Imaging: Advance Aims for Epilepsy’s Hidden Hot Spots
Posted on by Dr. Francis Collins
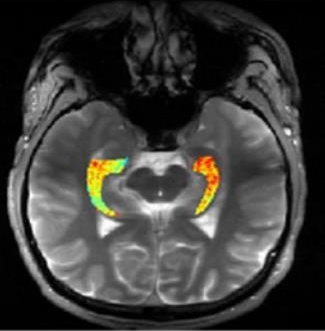
Credit: Reddy Lab, University of Pennsylvania
For many of the 65 million people around the world with epilepsy, modern medications are able to keep the seizures under control. When medications fail, as they do in about one-third of people with epilepsy, surgery to remove affected brain tissue without compromising function is a drastic step, but offers a potential cure. Unfortunately, not all drug-resistant patients are good candidates for such surgery for a simple reason: their brains appear normal on traditional MRI scans, making it impossible to locate precisely the source(s) of the seizures.
Now, in a small study published in Science Translational Medicine [1], NIH-funded researchers report progress towards helping such people. Using a new MRI method, called GluCEST, that detects concentrations of the nerve-signaling chemical glutamate in brain tissue [2], researchers successfully pinpointed seizure-causing areas of the brain in four of four volunteers with drug-resistant epilepsy and normal traditional MRI scans. While the findings are preliminary and must be confirmed by larger studies, researchers are hopeful that GluCEST, which takes about 30 minutes, may open the door to new ways of treating this type of epilepsy.
Epilepsy Research Benefits from the Crowd
Posted on by Dr. Francis Collins
 For millions of people with epilepsy, life comes with too many restrictions. If they just had a reliable way to predict when their next seizure will come, they could have a chance at leading more independent and productive lives.
For millions of people with epilepsy, life comes with too many restrictions. If they just had a reliable way to predict when their next seizure will come, they could have a chance at leading more independent and productive lives.
That’s why it is so encouraging to hear that researchers have developed a new algorithm that can predict the onset of a seizure correctly 82 percent of the time. Until recently, the best algorithm was hardly better than flipping a coin, leading some to speculate that seizures are random neurological events that can’t be predicted at all. But the latest leap forward shows that seizures certainly can be predicted, and our research efforts are headed in the right direction to make them even more predictable. The other big news is how this new algorithm was developed: it’s the product of a crowdsourcing competition.

