neglected tropical diseases
Creative Minds: Building a CRISPR Gene Drive Against Malaria
Posted on by Dr. Francis Collins

Valentino Gantz/Credit: Erik Jepsen
Researchers have used Drosophila melanogaster, the common fruit fly that sometimes hovers around kitchens, to make seminal discoveries involving genetics, the nervous system, and behavior, just to name a few. Could a new life-saving approach to prevent malaria be next? Valentino Gantz, a researcher at the University of California, San Diego, is on a path to answer that question.
Gantz has received a 2016 NIH Director’s Early Independence Award to use Drosophila to hone a new bioengineered tool that acts as a so-called “gene drive,” which spreads a new genetically encoded trait through a population much faster than would otherwise be possible. The lessons learned while working with flies will ultimately be applied to developing a more foolproof system for use in mosquitoes with the hope of stopping the transmission of malaria and potentially other serious mosquito-borne diseases.
Rare Disease Mystery: Nodding Syndrome May Be Linked to Parasitic Worm
Posted on by Dr. Francis Collins

Caption: Village in the East Africa nation of Uganda
Credit: Centers for Disease Control and Prevention
In the early 1960s, reports began to surface that some children living in remote villages in East Africa were suffering mysterious episodes of “head nodding.” The condition, now named nodding syndrome, is recognized as a rare and devastating form of epilepsy. There were hints that the syndrome might be caused by a parasitic worm called Onchocerca volvulus, which is transmitted through the bites of blackflies. But no one had been able to tie the parasitic infection directly to the nodding heads.
Now, NIH researchers and their international colleagues think they’ve found the missing link. The human immune system turns out to be a central player. After analyzing blood and cerebrospinal fluid of kids with nodding syndrome, they detected a particular antibody at unusually high levels [1]. Further studies suggest the immune system ramps up production of that antibody to fight off the parasite. The trouble is those antibodies also react against a protein in healthy brain tissue, apparently leading to progressive cognitive dysfunction, neurological deterioration, head nodding, and potentially life-threatening seizures.
The findings, published in Science Translational Medicine, have important implications for the treatment and prevention of not only nodding syndrome, but perhaps other autoimmune-related forms of epilepsy. As people in the United States and around the globe today observe the 10th anniversary of international Rare Disease Day, this work provides yet another example of how rare disease research can shed light on more common diseases and fundamental aspects of human biology.
Creative Minds: Interrogating a Master of Disguise
Posted on by Dr. Francis Collins
When I volunteered several years ago as a physician in a small hospital in West Africa, one of the most frustrating and frightening diseases I saw was sleeping sickness. Now, an investigator supported by the NIH Common Fund aims to figure out how this disease pathogen manages to evade the human immune system.
Monica Mugnier’s fascination with parasites started in college when she picked up the book Parasite Rex, a riveting, firsthand account of how “sneaky” parasites can be. The next year, while studying abroad in England, Mugnier met a researcher who had studied one of the most devious of parasites—a protozoan, spread by blood-sucking tsetse flies, that causes sleeping sickness in humans and livestock across sub-Saharan Africa.
Climate and Viral Illness: El Niño Event Linked to Dengue Epidemics
Posted on by Dr. Francis Collins
Just as the severity of the winter flu fluctuates from year to year in the United States, dengue fever can rage through tropical and subtropical regions of the world during their annual rainy seasons, causing potentially life-threatening high fever, severe joint pain, and bleeding. Other years—for still unknown reasons—dengue fizzles out. While many nations monitor the incidence of dengue within their borders, their data aren’t always combined to track outbreaks across wider regions over longer times.
Now, NIH-funded researchers and colleagues, reporting in Proceedings of the National Academy of Sciences [1], have linked an intense dengue epidemic that struck eight Southeast Asian countries starting in mid-1997 to high temperatures driven by the strongest El Niño event in recent times. El Niño is a complex, irregularly occurring series of climate changes in the Pacific Ocean with a global impact on weather patterns. This new insight into climatic factors associated with dengue transmission could enable better prevention measures, which may soon be needed because climatologists are predicting another strong El Niño event next year due to unusually high ocean temperatures in the equatorial Pacific.
Enlisting mHealth in the Fight Against River Blindness
Posted on by Dr. Francis Collins
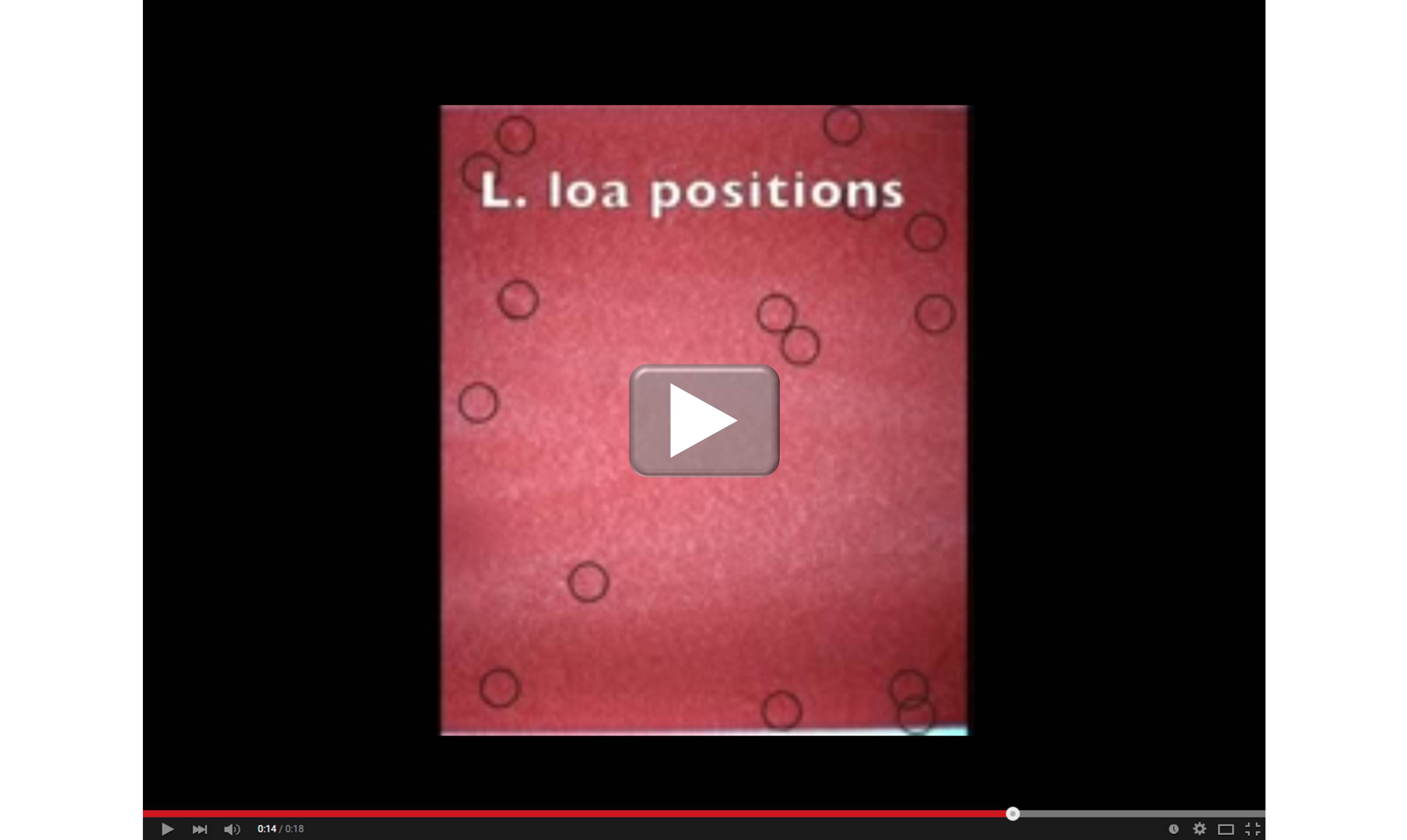
When it comes to devising new ways to provide state-of-the art medical care to people living in remote areas of the world, smartphones truly are helping scientists get smarter. For example, an NIH-supported team working in Central Africa recently turned an iPhone into a low-cost video microscope capable of quickly testing to see if people infected with a parasitic worm called Loa loa can safely receive a drug intended to protect them from a different, potentially blinding parasitic disease.
As shown in the video above, the iPhone’s camera scans a drop of a person’s blood for the movement of L. loa worms. Customized software then processes the motion to count the worms (see the dark circles) in the blood sample and arrive at an estimate of the body’s total worm load. The higher the worm load, the greater the risk of developing serious side effects from a drug treatment for river blindness, also known as onchocerciasis.