insulin
Artificial Pancreas Improves Blood Glucose Control in Young Kids with Type 1 Diabetes
Posted on by Lawrence Tabak, D.D.S., Ph.D.
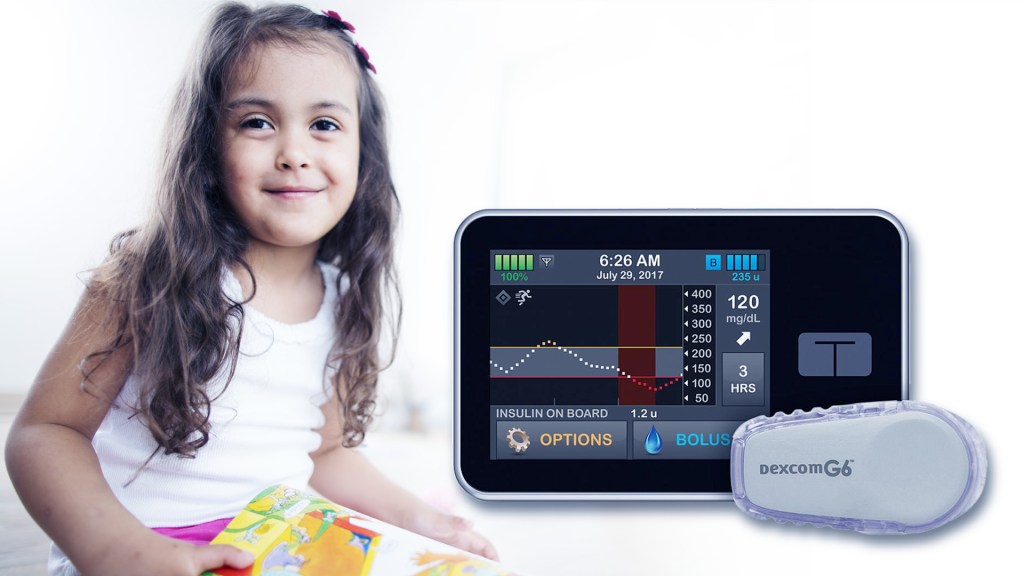
Last week brought some great news for parents of small children with type 1 diabetes (T1D). It involved what’s called an “artificial pancreas,” a new type of device to monitor continuously a person’s blood glucose levels and release the hormone insulin at the right time and at the right dosage, much like the pancreas does in kids who don’t have T1D.
Researchers published last week in the New England Journal of Medicine [1] the results of the largest clinical trial yet of an artificial pancreas technology in small children, ages 2 to 6. The data showed that their Control-IQ technology was safe and effective over several weeks at controlling blood glucose levels in these children. In fact, the new device performed better than the current standard of care.
Two previous clinical trials of the Control-IQ technology had shown the same in older kids and adults, age 6 and up [2,3], and the latest clinical trial, one of the first in young kids, should provide the needed data for the U. S. Food and Drug Administration (FDA) to consider whether to extend the age range approved to use this artificial pancreas. The FDA earlier approved two other artificial pancreas devices—the MiniMed 770G and the Insulet Omnipod 5 systems—for use in children age 2 and older [4,5].
The Control-IQ clinical trial results are a culmination of more than a decade-long effort by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and many others to create technologies, such as an artificial pancreas, to improve blood glucose control. The reason is managing blood glucose levels remains critical for the long-term health of people with T1D.
What exactly is an artificial pancreas? It consists of three fully integrated components: a glucose monitor, an insulin pump, and a computer algorithm that allows the other two components to communicate. This automation frees people with T1D from checking their blood glucose levels multiple times a day and from many insulin dosing decisions, though they still interact with the system at mealtimes.
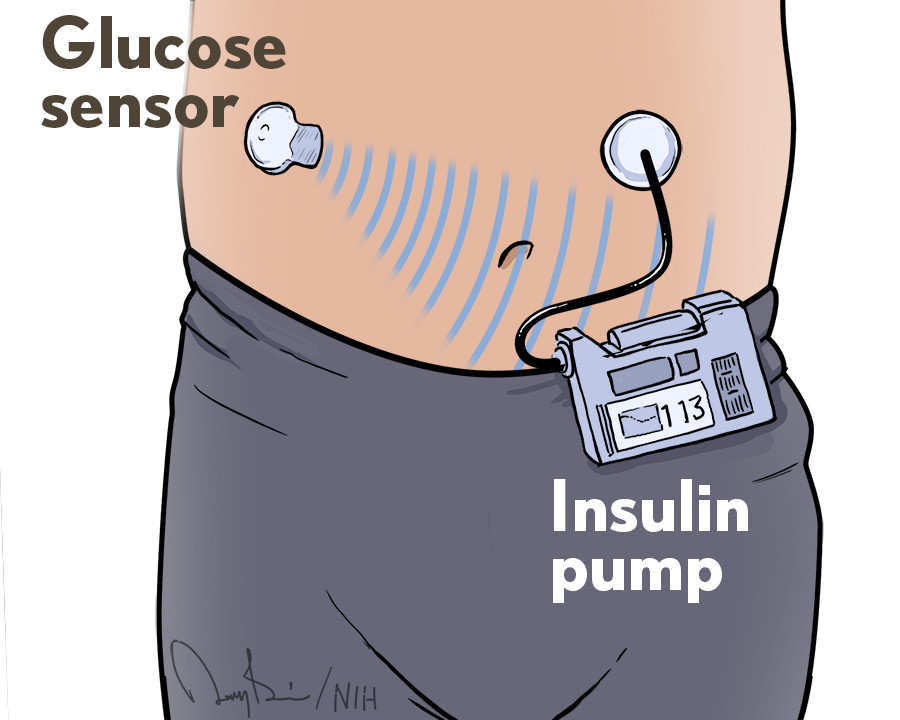
In this clinical trial, led by Marc D. Breton, University of Virginia School of Medicine, Charlottesville, researchers tested their Control-IQ technology (manufactured by Tandem Diabetes Care, San Diego, CA), also known as a hybrid closed-loop control system. Thanks to an algorithm developed at the University of Virginia Center for Diabetes Technology, insulin doses are administered automatically every few minutes based on readings from a continuous glucose monitor.
But treating younger children with T1D presents its own set of age-specific challenges. Younger kids generally require smaller doses of insulin more frequently. They also tend to have a more unpredictable schedule with lots of small snacks and random bursts of physical activity.
On top of all that, these young children have a tougher time than kids a few years older when it comes to understanding their own needs and letting the adults around them know when they need help. For all these reasons, young children with T1D tend to spend a greater proportion of time than older kids or adults do with blood glucose levels that are higher, or lower, than they should be. The hope was that the artificial pancreas might help to simplify things.
To find out, the trial enrolled 102 volunteers between ages 2 and 6. Sixty-eight were randomly assigned to receive the artificial pancreas, while the other 34 continued receiving insulin via either an insulin pump or multiple daily injections. The primary focus was on how long kids in each group spent in the target blood glucose range of 70 to 180 milligrams per deciliter, as measured using a continuous glucose monitor.
During the trial’s 13 weeks, participants in the artificial pancreas group spent approximately three more hours per day with their blood glucose in a healthy range compared to the standard care group. The greatest difference in blood glucose control was seen at night while the children should have been sleeping, from 10 p.m. to 6 a.m. During this important period, children with the artificial pancreas spent 18 percent more time in normal blood glucose range than the standard care group. That’s key because nighttime control is especially challenging to maintain in children with T1D.
Overall, the findings show benefits to young children similar to those seen previously in older kids. Those benefits also were observed in kids regardless of age, racial or ethnic group, parental education, or family income.
In the artificial pancreas group, there were two cases of severe hypoglycemia (low blood glucose) compared to one case in the other group. One child in the artificial pancreas group also developed diabetic ketoacidosis, a serious complication in which the body doesn’t have enough insulin. These incidents, while unfortunate, happened infrequently and at similar rates in the two groups.
Interestingly, the trial took place during the COVID-19 pandemic. As a result, much of the training on use of the artificial pancreas system took place virtually. Breton notes that the success of the artificial pancreas under these circumstances is an important finding, especially considering that many kids with T1D live in areas that are farther from endocrinologists or other specialists.
Even with these clinical trials now completed and a few devices on the market, there’s still more work to be done. The NIDDK has plans to host a meeting in the coming months to discuss next steps, including outstanding research questions and other priorities. It’s all very good news for people with T1D, including young kids and their families.
References:
[1] Trial of hybrid closed-loop control in young children with type 1 diabetes. Wadwa RP, Reed ZW, Buckingham BA, DeBoer MD, Ekhlaspour L, Forlenza GP, Schoelwer M, Lum J, Kollman C, Beck RW, Breton MD; PEDAP Trial Study Group. N Engl J Med. 2023 Mar 16;388(11):991-1001.
[2] A randomized trial of closed-loop control in children with type 1 diabetes. Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, Schoelwer M, Ruedy KJ, Jost E, Carria L, Emory E, Hsu LJ, Oliveri M, Kollman CC, Dokken BB, Weinzimer SA, DeBoer MD, Buckingham BA, Cherñavvsky D, Wadwa RP; iDCL Trial Research Group. N Engl J Med. 2020 Aug 27;383(9):836-845.
[3] Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, Laffel LM, Levy CJ, Pinsker JE, Wadwa RP, Dassau E, Doyle FJ 3rd, Anderson SM, Church MM, Dadlani V, Ekhlaspour L, Forlenza GP, Isganaitis E, Lam DW, Kollman C, Beck RW; N Engl J Med. 2019 Oct 31;381(18):1707-1717.
[4] MiniMed 770G System-P160017/S076. U. S. Food and Drug Administration, December 23, 2020.
[5] FDA authorizes Omnipod 5 for ages 2+ in children with type 1 diabetes. Juvenile Diabetes Research Foundation news release, August 22, 2022
Links:
Type I Diabetes (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Artificial Pancreas (NIDDK)
Marc Breton (University of Virginia, Charlottesville)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
How COVID-19 Can Lead to Diabetes
Posted on by Dr. Francis Collins

Along with the pneumonia, blood clots, and other serious health concerns caused by SARS-CoV-2, the COVID-19 virus, some studies have also identified another troubling connection. Some people can develop diabetes after an acute COVID-19 infection.
What’s going on? Two new NIH-supported studies, now available as pre-proofs in the journal Cell Metabolism [1,2], help to answer this important question, confirming that SARS-CoV-2 can target and impair the body’s insulin-producing cells.
Type 1 diabetes occurs when beta cells in the pancreas don’t secrete enough insulin to allow the body to metabolize food optimally after a meal. As a result of this insulin insufficiency, blood glucose levels go up, the hallmark of diabetes.
Earlier lab studies had suggested that SARS-CoV-2 can infect human beta cells [3]. They also showed that this dangerous virus can replicate in these insulin-producing beta cells, to make more copies of itself and spread to other cells [4].
The latest work builds on these earlier studies to discover more about the connection between COVID-19 and diabetes. The work involved two independent NIH-funded teams, one led by Peter Jackson, Stanford University School of Medicine, Palo Alto, CA, and the other by Shuibing Chen, Weill Cornell Medicine, New York. I’m actually among the co-authors on the study by the Chen team, as some of the studies were conducted in my lab at NIH’s National Human Genome Research Institute, Bethesda, MD.
Both studies confirmed infection of pancreatic beta cells in autopsy samples from people who died of COVID-19. Additional studies by the Jackson team suggest that the coronavirus may preferentially infect the insulin-producing beta cells.
This also makes biological sense. Beta cells and other cell types in the pancreas express the ACE2 receptor protein, the TMPRSS2 enzyme protein, and neuropilin 1 (NRP1), all of which SARS-CoV-2 depends upon to enter and infect human cells. Indeed, the Chen team saw signs of the coronavirus in both insulin-producing beta cells and several other pancreatic cell types in the studies of autopsied pancreatic tissue.
The new findings also show that the coronavirus infection changes the function of islets—the pancreatic tissue that contains beta cells. Both teams report evidence that infection with SARS-CoV-2 leads to reduced production and release of insulin from pancreatic islet tissue. The Jackson team also found that the infection leads directly to the death of some of those all-important beta cells. Encouragingly, they showed this could avoided by blocking NRP1.
In addition to the loss of beta cells, the infection also appears to change the fate of the surviving cells. Chen’s team performed single-cell analysis to get a careful look at changes in the gene activity within pancreatic cells following SARS-CoV-2 infection. These studies showed that beta cells go through a process of transdifferentiation, in which they appeared to get reprogrammed.
In this process, the cells begin producing less insulin and more glucagon, a hormone that encourages glycogen in the liver to be broken down into glucose. They also began producing higher levels of a digestive enzyme called trypsin 1. Importantly, they also showed that this transdifferentiation process could be reversed by a chemical (called trans-ISRIB) known to reduce an important cellular response to stress.
The consequences of this transdifferentiation of beta cells aren’t yet clear, but would be predicted to worsen insulin deficiency and raise blood glucose levels. More study is needed to understand how SARS-CoV-2 reaches the pancreas and what role the immune system might play in the resulting damage. Above all, this work provides yet another reminder of the importance of protecting yourself, your family members, and your community from COVID-19 by getting vaccinated if you haven’t already—and encouraging your loved ones to do the same.
References:
[1] SARS-CoV-2 infection induces beta cell transdifferentiation. Tang et al. Cell Metab 2021 May 19;S1550-4131(21)00232-1.
[2] SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Wu et al. Cell Metab. 2021 May 18;S1550-4131(21)00230-8.
[3] A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Yang L, Han Y, Nilsson-Payant BE, Evans T, Schwartz RE, Chen S, et al. Cell Stem Cell. 2020 Jul 2;27(1):125-136.e7.
[4] SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Müller JA, Groß R, Conzelmann C, Münch J, Heller S, Kleger A, et al. Nat Metab. 2021 Feb;3(2):149-165.
Links:
COVID-19 Research (NIH)
Type 1 Diabetes (National Institute of Diabetes, Digestive and Kidney Disorders/NIH)
Jackson Lab (Stanford Medicine, Palo Alto, CA)
Shuibing Chen Laboratory (Weill Cornell Medicine, New York City)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases; National Human Genome Research Institute; National Institute of General Medical Sciences; National Cancer Institute; National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development
Insulin-Producing Organoids Offer Hope for Treating Type 1 Diabetes
Posted on by Dr. Francis Collins

For the 1 to 3 million Americans with type 1 diabetes, the immune system destroys insulin-producing beta cells of the pancreas that control the amount of glucose in the bloodstream. As a result, these individuals must monitor their blood glucose often and take replacement doses of insulin to keep it under control. Such constant attention, combined with a strict diet to control sugar intake, is challenging—especially for children.
For some people with type 1 diabetes, there is another option. They can be treated—maybe even cured—with a pancreatic islet cell transplant from an organ donor. These transplanted islet cells, which harbor the needed beta cells, can increase insulin production. But there’s a big catch: there aren’t nearly enough organs to go around, and people who receive a transplant must take lifelong medications to keep their immune system from rejecting the donated organ.
Now, NIH-funded scientists, led by Ronald Evans of the Salk Institute, La Jolla, CA, have devised a possible workaround: human islet-like organoids (HILOs) [1]. These tiny replicas of pancreatic tissue are created in the laboratory, and you can see them above secreting insulin (green) in a lab dish. Remarkably, some of these HILOs have been outfitted with a Harry Potter-esque invisibility cloak to enable them to evade immune attack when transplanted into mice.
Over several years, Doug Melton’s lab at Harvard University, Cambridge, MA, has worked steadily to coax induced pluripotent stem (iPS) cells, which are made from adult skin or blood cells, to form miniature islet-like cells in a lab dish [2]. My own lab at NIH has also been seeing steady progress in this effort, working with collaborators at the New York Stem Cell Foundation.
Although several years ago researchers could get beta cells to make insulin, they wouldn’t secrete the hormone efficiently when transplanted into a living mouse. About four years ago, the Evans lab found a possible solution by uncovering a genetic switch called ERR-gamma that when flipped, powered up the engineered beta cells to respond continuously to glucose and release insulin [3].
In the latest study, Evans and his team developed a method to program HILOs in the lab to resemble actual islets. They did it by growing the insulin-producing cells alongside each other in a gelatinous, three-dimensional chamber. There, the cells combined to form organoid structures resembling the shape and contour of the islet cells seen in an actual 3D human pancreas. After they are switched on with a special recipe of growth factors and hormones, these activated HILOs secrete insulin when exposed to glucose. When transplanted into a living mouse, this process appears to operate just like human beta cells work inside a human pancreas.
Another major advance was the invisibility cloak. The Salk team borrowed the idea from cancer immunotherapy and a type of drug called a checkpoint inhibitor. These drugs harness the body’s own immune T cells to attack cancer. They start with the recognition that T cells display a protein on their surface called PD-1. When T cells interact with other cells in the body, PD-1 binds to a protein on the surface of those cells called PD-L1. This protein tells the T cells not to attack. Checkpoint inhibitors work by blocking the interaction of PD-1 and PD-L1, freeing up immune cells to fight cancer.
Reversing this logic for the pancreas, the Salk team engineered HILOs to express PD-L1 on their surface as a sign to the immune system not to attack. The researchers then transplanted these HILOs into diabetic mice that received no immunosuppressive drugs, as would normally be the case to prevent rejection of these human cells. Not only did the transplanted HILOs produce insulin in response to glucose spikes, they spurred no immune response.
So far, HILOs transplants have been used to treat diabetes for more than 50 days in diabetic mice. More research will be needed to see whether the organoids can function for even longer periods of time.
Still, this is exciting news, and provides an excellent example of how advances in one area of science can provide new possibilities for others. In this case, these insights provide fresh hope for a day when children and adults with type 1 diabetes can live long, healthy lives without the need for frequent insulin injections.
References:
[1] Immune-evasive human islet-like organoids ameliorate diabetes. [published online ahead of print, 2020 Aug 19]. Yoshihara E, O’Connor C, Gasser E, Wei Z, Oh TG, Tseng TW, Wang D, Cayabyab F, Dai Y, Yu RT, Liddle C, Atkins AR, Downes M, Evans RM. Nature. 2020 Aug 19. [Epub ahead of publication]
[2] Generation of Functional Human Pancreatic β Cells In Vitro. Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Cell. 2014 Oct 9;159(2):428-39.
[3] ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, Atkins AR, Downes M, Evans RM. Cell Metab. 2016 Apr 12; 23(4):622-634.
Links:
Type 1 Diabetes (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Pancreatic Islet Transplantation (National Institute of Diabetes and Digestive and Kidney Diseases)
“The Nobel Prize in Physiology or Medicine 2012” for Induced Pluripotent Stem Cells, The Nobel Prize news release, October 8, 2012.
Evans Lab (Salk Institute, La Jolla, CA)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases; National Cancer Institute
Oral Insulin Delivery: Can the Tortoise Win the Race?
Posted on by Dr. Francis Collins
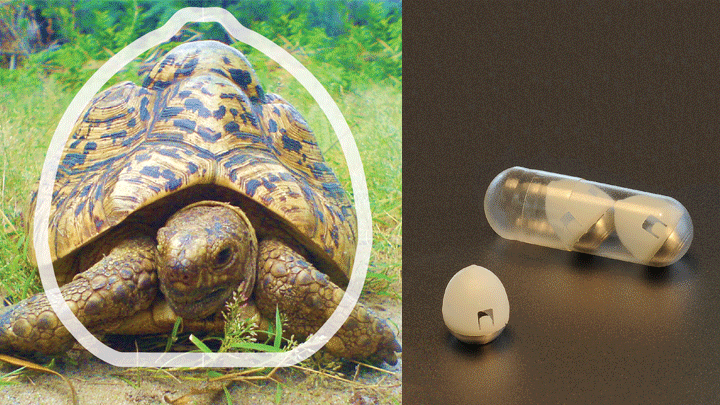
People with diabetes often must inject insulin multiple times a day to keep their blood glucose levels under control. So, I was intrigued to learn that NIH-funded bioengineers have designed a new kind of “pill” that may someday reduce the need for those uncomfortable shots. The inspiration for their design? A tortoise!
The new “pill”—actually, a swallowable device containing a tiny injection system—is shaped like the shell of an African leopard tortoise. In much the same way that the animal’s highly curved shell enables it to quickly right itself when flipped on its back, the shape of the new device is intended to help it land in the right position to inject insulin or other medicines into the stomach wall.
The hunt for a means to deliver insulin in pill form has been on ever since insulin injections first were introduced, nearly a century ago. The challenge in oral delivery of insulin and other “biologic” drugs—including therapeutic proteins, peptides, or nucleic acids—is how to get these large biomolecules through the highly acidic stomach and duodenum, where multiple powerful digestive enzymes reside, and into the bloodstream unscathed. Past efforts to address this challenge have met with only limited success.
In a study published in the journal Science, a team, led by Robert Langer at Massachusetts Institute of Technology, Cambridge, and Giovanni Traverso, Brigham and Women’s Hospital, Harvard Medical School, Boston, took a new approach to the problem by developing a tiny, ingestible injection system [1]. They call their pea-sized device SOMA, short for “self-orienting millimeter-scale applicator.”
In designing SOMA, the researchers knew they had to come up with a design that would orient the injection apparatus correctly. So they looked to the African leopard tortoise. They knew that, much like a child’s “weeble-wobble” toy, this tortoise can easily right its body if tipped over due to its low center of gravity and highly curved shell. With the shape of the tortoise shell as a starting point, the researchers used computer modeling to perfect their design. The final result features a partially hollowed-out, polymer-and-steel capsule that houses a tiny, spring-loaded needle tipped with compressed, freeze-dried insulin. There is also a dissolvable sugar disk to hold the needle in place until the time is right.
Here’s how it works: once a SOMA is swallowed and reaches the stomach, it quickly orients itself in a way that its needle-side rests against the stomach wall. After the protective sugar disk dissolves in stomach acid, the spring-loaded needle tipped with insulin is released, injecting its load of insulin into the stomach wall, from which it enters the bloodstream. Meanwhile, the spent SOMA device passes on through the digestive system.
The researchers’ tests in pigs have shown that a single SOMA can successfully deliver insulin doses of up to 3 milligrams, comparable to the amount a human with diabetes might need to inject. The tests also showed that the device’s microinjection did not damage the animals’ stomach tissue or the muscles surrounding the stomach. Because the stomach is known for being insensitive to pain, researchers expect that people receiving insulin via SOMA wouldn’t feel a thing, but much more research is needed to confirm both the safety and efficacy of the new device for human use.
Meanwhile, this fascinating work serves as a reminder that when it comes to biomedical science, inspiration sometimes can come from the most unexpected places.
Reference:
[1] An ingestible self-orienting system for oral delivery of macromolecules. Abramson A, Caffarel-Salvador E, Khang M, Dellal D, Silverstein D, Gao Y, Frederiksen MR, Vegge A, Hubálek F, Water JJ, Friderichsen AV, Fels J, Kirk RK, Cleveland C, Collins J, Tamang S, Hayward A, Landh T, Buckley ST, Roxhed N, Rahbek U, Langer R, Traverso G. Science. 2019 Feb 8;363(6427):611-615.
Links:
Diabetes (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Langer Lab (MIT, Cambridge)
Giovanni Traverso (Brigham and Women’s Hospital, Harvard Medical School, Boston)
NIH Support: National Institute of Biomedical Imaging and Bioengineering
Can Artificial Cells Take Over for Lost Insulin-Secreting Cells?
Posted on by Dr. Francis Collins

Caption: Artificial beta cell, made of a lipid bubble (purple) carrying smaller, insulin-filled vesicles (green). Imaged with cryo-scanning electron microscope (cryo-SEM) and colorized.
Credit: Zhen Gu Lab
People with diabetes have benefited tremendously from advances in monitoring and controlling blood sugar, but they’re still waiting and hoping for a cure. Some of the most exciting possibilities aim to replace the function of the insulin-secreting pancreatic beta cells that is deficient in diabetes. The latest strategy of this kind is called AβCs, short for artificial beta cells.
As you see in the cryo-SEM image above, AβCs are specially designed lipid bubbles, each of which contains hundreds of smaller, ball-like vesicles filled with insulin. The AβCs are engineered to “sense” a rise in blood glucose, triggering biochemical changes in the vesicle and the automatic release of some of its insulin load until blood glucose levels return to normal.
In recent studies of mice with type 1 diabetes, researchers partially supported by NIH found that a single injection of AβCs under the skin could control blood glucose levels for up to five days. With additional optimization and testing, the hope is that people with diabetes may someday be able to receive AβCs through patches that painlessly stick on their skin.
Cool Videos: Insulin from Bacteria to You
Posted on by Dr. Francis Collins
If you have a smartphone, you’ve probably used it to record a video or two. But could you use it to produce a video that explains a complex scientific topic in 2 minutes or less? That was the challenge posed by the RCSB Protein Data Bank last spring to high school students across the nation. And the winning result is the video that you see above!
This year’s contest, which asked students to provide a molecular view of diabetes treatment and management, attracted 53 submissions from schools from coast to coast. The winning team—Andrew Ma, George Song, and Anirudh Srikanth—created their video as their final project for their advanced placement (AP) biology class at West Windsor-Plainsboro High School South, Princeton Junction, NJ.
Blood Sugar Control for Diabetes: Asking the Heart Questions
Posted on by Dr. Francis Collins
When most people think about risk factors for cardiovascular disease, they likely think of blood pressure readings or cholesterol levels. But here’s something else that should be high on that list: diabetes. That’s because people with diabetes are roughly twice as likely to die of heart disease than other folks [1]. Yet the issue of how best to help such people lower their cardiovascular risks remains a matter of intense debate. Some studies have suggested that part of the answer may lie in tightly controlling blood sugar (glucose) levels with a strict regimen of medications and monitoring [2]. Other research has shown that the intense effort needed to keep blood glucose levels under tight control might not be worth it and may even make things worse for certain individuals [3].
Now, a follow up of a large, clinical trial involving nearly 1,800 U.S. military veterans with type 2 diabetes—the most common form of diabetes—provides further evidence that tight blood glucose control may indeed protect the cardiovascular system. Reporting in The New England Journal of Medicine [4], researchers found a significant reduction in a composite measure of heart attacks, strokes, heart failure, and circulation-related amputations among the vets who maintained tight glucose control for about five and a half years on average. What’s particularly encouraging is most of the cardiovascular-protective benefit appears to be achievable through relatively modest, rather than super strict, reductions in blood glucose levels.
Stem Cell Science: Taking Aim at Type 1 Diabetes
Posted on by Dr. Francis Collins
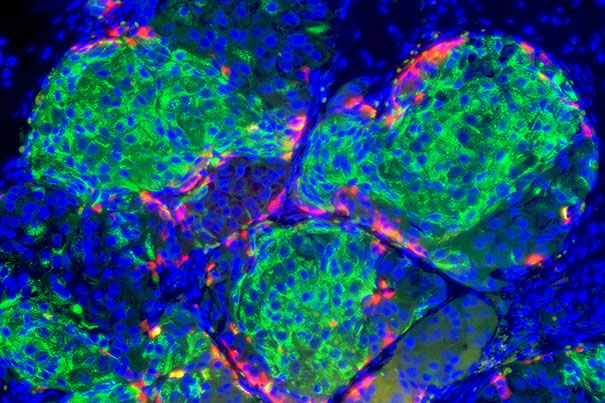
Caption: Insulin-producing pancreatic beta cells (green) derived from human embryonic stem cells that have formed islet-like clusters in a mouse. The red cells are producing another metabolic hormone, glucagon, that regulates blood glucose levels. Blue indicates cell nuclei.
Credit: Photo by B. D. Colen/Harvard Staff; Image courtesy of Doug Melton
For most of the estimated 1 to 3 million Americans living with type 1 diabetes, every day brings multiple fingerpricks to manage their blood glucose levels with replacement insulin [1,2]. The reason is that their own immune systems have somehow engaged in friendly fire on small, but vital, clusters of cells in the pancreas known as the islets—which harbor the so-called “beta cells” that make insulin. So, it’s no surprise that researchers seeking ways to help people with type 1 diabetes have spent decades trying a find a reliable way to replace these islets.
Islet replacement has proven to be an extremely difficult research challenge for a variety of reasons, but exciting opportunities are now on the horizon. Notably, a team of researchers, led by Douglas Melton of Harvard University, Cambridge, MA, and partially funded by NIH, reported groundbreaking success just last week in spurring a human embryonic stem cell (hESC) line and two human-induced pluripotent stem (iPS) cell lines to differentiate into the crucial, insulin-producing beta cells. Not only did cells generated from all three of these lines look like human pancreatic beta cells, they functioned like bona fide, glucose-responsive beta cells in a mouse model of type 1 diabetes [3].
Next Page



