Stem Cell Science: Taking Aim at Type 1 Diabetes
Posted on by Dr. Francis Collins
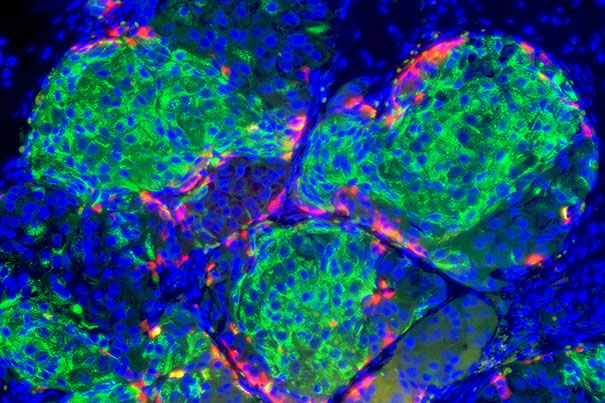
Caption: Insulin-producing pancreatic beta cells (green) derived from human embryonic stem cells that have formed islet-like clusters in a mouse. The red cells are producing another metabolic hormone, glucagon, that regulates blood glucose levels. Blue indicates cell nuclei.
Credit: Photo by B. D. Colen/Harvard Staff; Image courtesy of Doug Melton
For most of the estimated 1 to 3 million Americans living with type 1 diabetes, every day brings multiple fingerpricks to manage their blood glucose levels with replacement insulin [1,2]. The reason is that their own immune systems have somehow engaged in friendly fire on small, but vital, clusters of cells in the pancreas known as the islets—which harbor the so-called “beta cells” that make insulin. So, it’s no surprise that researchers seeking ways to help people with type 1 diabetes have spent decades trying a find a reliable way to replace these islets.
Islet replacement has proven to be an extremely difficult research challenge for a variety of reasons, but exciting opportunities are now on the horizon. Notably, a team of researchers, led by Douglas Melton of Harvard University, Cambridge, MA, and partially funded by NIH, reported groundbreaking success just last week in spurring a human embryonic stem cell (hESC) line and two human-induced pluripotent stem (iPS) cell lines to differentiate into the crucial, insulin-producing beta cells. Not only did cells generated from all three of these lines look like human pancreatic beta cells, they functioned like bona fide, glucose-responsive beta cells in a mouse model of type 1 diabetes [3].
Creating a stockpile of insulin-secreting beta cells has been a longstanding need for many areas of diabetes research, particularly for the still-experimental field of islet transplantation. Currently, such transplants are performed using pancreatic cells harvested from deceased donors (a procedure somewhat akin to heart, liver, or other transplants). Despite some reports of islet transplantation curing type 1 diabetes for five years or more, one major obstacle on the road to wider clinical use is the extremely limited supply of transplantable islets.
Fifteen years ago, one intriguing possibility for meeting this supply problem emerged when basic scientists isolated the first hESCs. Like gardeners learning to germinate a new type of seed, researchers worldwide began tinkering with which growth-inducing factors to add, when, and in which sequence, to prompt these undifferentiated stem cells to commit to becoming specialized adult cells. Successes began to appear for cardiac cells, neurons, smooth muscle cells, and other cell types, but beta cells proved to be extremely challenging. Furthermore, just a few cells wouldn’t do: to be useful, the protocol would have to produce a vast, renewable source of functional beta cells for transplantation. And what a vast source that would have to be! It takes anywhere from 340 million to 750 million beta cells to provide treatment for type 1 diabetes in just one 150-pound person.
What’s particularly encouraging about the latest findings is they suggest a way forward for solving the supply problem. After years of trial and error, Melton and his colleagues have developed a rigorous recipe of adding and subtracting natural biological factors to coax stem cell lines into becoming functional, insulin-secreting pancreatic beta cells. Within a matter of a few weeks, this approach can reliably produce 300 million beta cells in a single 500 milliliter flask—with researchers estimating that one or two flasks of such cells would be enough to treat a patient. If these results, which were published in the journal Cell, can be reproduced and extended to humans, the ability to generate an ample supply of fully functional, insulin-producing beta cells may finally be within our reach.
Melton’s team has not been alone in this multiyear effort—many other researchers in the public and private sectors have been hard at work developing potential stem-cell therapies for type 1 diabetes and other diseases. However, I was especially pleased to learn that the hESC line used in their work (HUES8) was among the first lines approved by NIH in response to a 2009 Executive Order enabling more hESC lines to be added to the list of those approved for federally funded research. In accordance with NIH guidelines, HUES8 was derived from embryos that were donated under ethically sound informed consent processes. The other cell lines used in this study are induced pluripotent stem (iPS) cell lines, which are reprogrammed adult skin or blood cells with the ability to differentiate into heart, nerve, muscle, and many other kinds of cells. iPS cell technology builds upon the Nobel Prize-winning work of Japan’s Shinya Yamanaka, and iPS cells have the particularly appealing feature of being obtainable from anyone, meaning a supply of iPS-derived beta cells could be potentially produced and reinfused without risk of transplant rejection.
For all who have type 1 diabetes or children with the disease (and Melton has two!), the most recent stem cell research accomplishment, while exciting, is still incomplete until it makes a real difference in patients’ lives. To that end, Melton and his colleagues are now collaborating to develop an implantable micro-device, about the size of a credit card, that will shield transplants of insulin-producing beta cells from that unwanted friendly fire of the immune system—another major hurdle that must be cleared in order for such treatment approaches to become commonplace [4]. If researchers succeed in developing this and other next-generation treatments, we’d certainly have cause to celebrate—not only because of the excellent science but, most of all, the improved quality of life and potential longer term survival for those who live each day with type 1 diabetes.
References:
[1] Foundational Data Report: The Size of the Population Impacted by Type 1 Diabetes. Juvenile Diabetes Cure Alliance, 2013.
[2] National Diabetes Statistics Report, 2014. Centers for Disease Control and Prevention.
[3] Generation of Functional Human Pancreatic β Cells In Vitro. Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Cell. 2014 Oct 9;159(2):428-39.
[4] Stem-cell Success Poses Immunity Challenge for Diabetes. Ledford H. Nature 2014. Oct 14.
Links:
Stem Cell Information (NIH)
Your Guide to Diabetes: Type 1 and Type 2 (NIDDK/NIH)
Melton Laboratory, Harvard University
NIH support: National Institute of Diabetes and Digestive and Kidney Diseases

Dear Dr. Francis Collins ,
please make a video or other digital method for “ebola screening and precaution protocol” that can be sent to every state for use by all doctors and nurses and facilities and accessed by airports, docks, points of entry, and everyone- for immediate use! in liberia, they take childrens temps before school each day. what are we waiting for. God bless you all.