viral pandemics
The Prime Cellular Targets for the Novel Coronavirus
Posted on by Dr. Francis Collins

There’s still a lot to learn about SARS-CoV-2, the novel coronavirus that causes COVID-19. But it has been remarkable and gratifying to watch researchers from around the world pull together and share their time, expertise, and hard-earned data in the urgent quest to control this devastating virus.
That collaborative spirit was on full display in a recent study that characterized the specific human cells that SARS-CoV-2 likely singles out for infection [1]. This information can now be used to study precisely how each cell type interacts with the virus. It might ultimately help to explain why some people are more susceptible to SARS-CoV-2 than others, and how exactly to target the virus with drugs, immunotherapies, and vaccines to prevent or treat infections.
This work was driven by the mostly shuttered labs of Alex K. Shalek, Massachusetts Institute of Technology, Ragon Institute of MGH, MIT, and Harvard, and Broad Institute of MIT and Harvard, Cambridge; and Jose Ordovas-Montanes at Boston Children’s Hospital. In the end, it brought together (if only remotely) dozens of their colleagues in the Human Cell Atlas Lung Biological Network and others across the U.S., Europe, and South Africa.
The project began when Shalek, Ordovas-Montanes, and others read that before infecting human cells, SARS-CoV-2 docks on a protein receptor called angiotensin-converting enzyme 2 (ACE2). This enzyme plays a role in helping the body maintain blood pressure and fluid balance.
The group was intrigued, especially when they also learned about a second enzyme that the virus uses to enter cells. This enzyme goes by the long acronym TMPRSS2, and it gets “tricked” into priming the spike proteins that cover SARS-CoV-2 to attack the cell. It’s the combination of these two proteins that provide a welcome mat for the virus.
Shalek, Ordovas-Montanes, and an international team including graduate students, post-docs, staff scientists, and principal investigators decided to dig a little deeper to find out precisely where in the body one finds cells that express this gene combination. Their curiosity took them to the wealth of data they and others had generated from model organisms and humans, the latter as part of the Human Cell Atlas. This collaborative international project is producing a comprehensive reference map of all human cells. For its first draft, the Human Cell Atlas aims to gather information on at least 10 billion cells.
To gather this information, the project relies, in part, on relatively new capabilities in sequencing the RNA of individual cells. Keep in mind that every cell in the body has essentially the same DNA genome. But different cells use different programs to decide which genes to turn on—expressing those as RNA molecules that can be translated into protein. The single-cell analysis of RNA allows them to characterize the gene expression and activities within each and every unique cell type. Based on what was known about the virus and the symptoms of COVID-19, the team focused their attention on the hundreds of cell types they identified in the lungs, nasal passages, and intestines.
As reported in Cell, by filtering through the data to identify cells that express ACE2 and TMPRSS2, the researchers narrowed the list of cell types in the nasal passages down to the mucus-producing goblet secretory cells. In the lung, evidence for activity of these two genes turned up in cells called type II pneumocytes, which line small air sacs known as alveoli and help to keep them open. In the intestine, it was the absorptive enterocytes, which play an important role in the body’s ability to take in nutrients.
The data also turned up another unexpected and potentially important connection. In these cells of interest, all of which are found in epithelial tissues that cover or line body surfaces, the ACE2 gene appeared to ramp up its activity in concert with other genes known to respond to interferon, a protein that the body makes in response to viral infections.
To dig further in the lab, the researchers treated cultured cells that line airways in the lungs with interferon. And indeed, the treatment increased ACE2 expression.
Earlier studies have suggested that ACE2 helps the lungs to tolerate damage. Completely missed was its connection to the interferon response. The researchers now suspect that’s because it hadn’t been studied in these specific human epithelial cells before.
The discovery suggests that SARS-CoV-2 and potentially other coronaviruses that rely on ACE2 may take advantage of the immune system’s natural defenses. When the body responds to the infection by producing more interferon, that in turn results in production of more ACE2, enhancing the ability of the virus to attach more readily to lung cells. While much more work is needed, the finding indicates that any potential use of interferon as a treatment to fight COVID-19 will require careful monitoring to determine if and when it might help patients.
It’s clear that these new findings, from data that weren’t originally generated with COVID-19 in mind, contained several potentially important new leads. This is another demonstration of the value of basic science. We can also rest assured that, with the outpouring of effort from members of the scientific community around the globe to meet this new challenge, progress along these and many other fronts will continue at a remarkable pace.
Reference:
[1] SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Ziegler, CGK et al. Cell. April 20, 2020.
Links:
Coronaviruses (National Institute of Allergy and Infectious Diseases/NIH)
Human Cell Atlas (Broad Institute, Cambridge, MA)
Shalek Lab (Harvard Medical School and Massachusetts Institute of Technology, Cambridge)
Ordovas-Montanes Lab (Boston Children’s Hospital, MA)
NIH Support: National Institute of Allergy and Infectious Diseases; National Institute of General Medical Sciences; National Heart, Lung, and Blood Institute
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: ACE2, alveoli, basic research, COVID-19, enterocytes, epithelial cells, gene expression, genomics, goblet cells, Human Cell Atlas, Human Cell Atlas Lung Biological Network, infectious diseases, interferon, intestine, lungs, nasal passage, novel coronavirus, pandemic, RNA, SARS-CoV-2, single cell analysis, spike protein, TMPRSS2, type II pneumocytes, viral pandemics
Antibody Points to Possible Weak Spot on Novel Coronavirus
Posted on by Dr. Francis Collins

Researchers are working hard to produce precise, 3D molecular maps to guide the development of safe, effective ways of combating the coronavirus disease 2019 (COVID-19) pandemic. While there’s been a lot of excitement surrounding the promise of antibody-based tests and treatments, this map you see above highlights another important use of antibodies: to inform efforts to design a vaccine.
This image shows the crystal structure of a human antibody (heavy chain in orange, light chain in yellow), which is a blood protein our immune systems produce to attack viruses and other foreign invaders. This particular antibody, called CR3022, is bound to a key surface protein of the novel coronavirus (white).
The CR3022 antibody actually doesn’t come from someone who has recovered from COVID-19. Instead, it was obtained from a person who, nearly two decades ago, survived a bout of severe acute respiratory syndrome (SARS). The SARS virus, which disappeared in 2004 after a brief outbreak in humans, is closely related to the novel coronavirus that causes COVID-19.
In a recent paper in the journal Science, the NIH-funded lab of Ian Wilson, The Scripps Research Institute, La Jolla, CA, along with colleagues at The University of Hong Kong, sought to understand how the human immune system interacts with and neutralizes this highly infectious virus [1]. The lab did so by employing high-resolution X-ray crystallography tools [2]. They captured the atomic structure of this antibody bound to its target by shooting X-rays through its crystallized form. (An antibody measures about 10 nanometers; a nanometer is 1 billionth of a meter.)
Other researchers had shown previously that CR3022 cross-reacts with the novel coronavirus, although the antibody doesn’t bind tightly enough to neutralize and stop it from infecting cells. So, Wilson’s team went to work to learn precisely where the antibody attaches to the novel virus. Those sites are of special interest because they highlight spots on a virus that are vulnerable to attack—and, as such, potentially good targets for vaccine designers.
A key finding in the new paper is that the antibody binds a highly similar site on both the SARS and novel coronaviruses. Those sites differ in each virus by just four amino acids, the building blocks of a protein.
This is particularly interesting because the antibody pictured above is bound to a spike protein, which is the appendage on both the SARS and novel coronavirus that enables them to bind to a key receptor protein on the surface of human cells, called ACE2. This binding activity marks the first step for these viruses in gaining entry into human cells and infecting them.
The human antibody shown in this image locks onto the virus’s spike protein at a different location than where the human ACE2 protein binds to the novel coronavirus. Intriguingly, the antibody binds to a spot on the novel coronavirus that is usually hidden, except for when virus shapeshifts its structure in order to infect a cell.
The findings suggest that a successful vaccine may be one that elicits antibodies that targets this same spot, but binds more tightly than the one seen above, thereby protecting human cells against the virus that causes COVID-19. However, Wilson notes that this study has just uncovered one potential vulnerability of the novel coronavirus, and it is likely the virus likely has many more that could be revealed with further study.
To continue in this quest to design a safe and effective vaccine, Wilson and his colleagues are now gathering blood samples to collect antibodies from people who’ve recovered from COVID-19. So, we can look forward to seeing some even more revealing images soon.
References:
[1] A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Yuan M, Wu NC, Zhu X, Lee CD, So RTY, Lv H, Mok CKP, Wilson IA. Science. 2020 Apr 3.
[2] 100 Years Later: Celebrating the Contributions of X-ray Crystallography to Allergy and Clinical Immunology. Pomés A, Chruszcz M, Gustchina A, Minor W, Mueller GA, Pedersen LC, Wlodawer A, Chapman MD. J Allergy Clin Immunol. 2015 Jul;136(1):29-37.
Links:
Coronaviruses (National Institute of Allergy and Infectious Diseases/NIH)
Coronavirus (COVID-19) (NIH)
Ian Wilson (The Scripps Research Institute, La Jolla, CA)
NIH Support: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Institute of General Medical Sciences
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Bringing Needed Structure to COVID-19 Drug Development
Posted on by Dr. Francis Collins
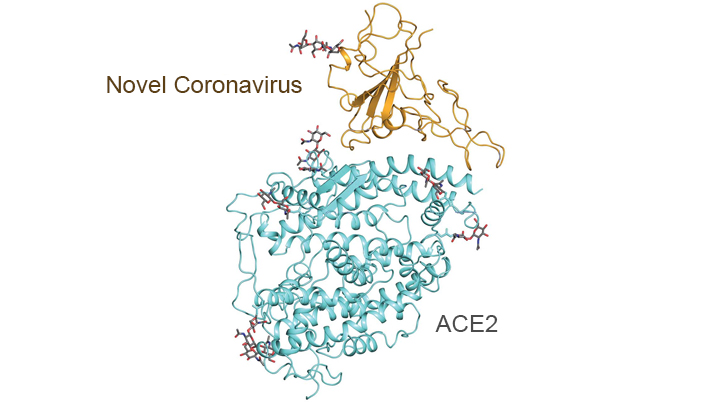
With so much information swirling around these days about the coronavirus disease 2019 (COVID-19) pandemic, it would be easy to miss one of the most interesting and significant basic science reports of the past few weeks. It’s a paper published in the journal Science [1] that presents an atomic-scale snapshot showing the 3D structure of the spike protein on the novel coronavirus attached to a human cell surface protein called ACE2, or angiotensin converting enzyme 2. ACE2 is the receptor that the virus uses to gain entry.
What makes this image such a big deal is that it shows—in exquisite detail—how the coronavirus attaches to human cells before infecting them and making people sick. The structural map of this interaction will help guide drug developers, atom by atom, in devising safe and effective ways to treat COVID-19.
This new work, conducted by a team led by Qiang Zhou, Westlake Institute for Advanced Study, Hangzhou, China, took advantage of a high-resolution imaging tool called cryo-electron microscopy (cryo-EM). This approach involves flash-freezing molecules in liquid nitrogen and bombarding them with electrons to capture their images with a special camera. When all goes well, cryo-EM can solve the structure of intricate macromolecular complexes in a matter of days, including this one showing the interaction between a viral protein and human protein.
Zhou’s team began by mapping the structure of human ACE2 in a complex with B0AT1, which is a membrane protein that it helps to fold. In the context of this complex, ACE2 is a dimer—a scientific term for a compound composed of two very similar units. Additional mapping revealed how the surface protein of the novel coronavirus interacts with ACE2, indicating how the virus’s two trimeric (3-unit) spike proteins might bind to an ACE2 dimer. After confirmation by further research, these maps may well provide a basis for the design and development of therapeutics that specifically target this critical interaction.
The ACE2 protein resides on the surface of cells in many parts of the human body, including the heart and lungs. The protein is known to play a prominent role in the body’s complex system of regulating blood pressure. In fact, a class of drugs that inhibit ACE and related proteins are frequently prescribed to help control high blood pressure, or hypertension. These ACE inhibitors lower blood pressure by causing blood vessels to relax.
Since the COVID-19 outbreak, many people have wondered whether taking ACE inhibitors would be helpful or detrimental against coronavirus infection. This is of particular concern to doctors whose patients are already taking the medications to control hypertension. Indeed, data from China and elsewhere indicate hypertension is one of several coexisting conditions that have consistently been reported to be more common among people with COVID-19 who develop life-threatening severe acute respiratory syndrome.
In a new report in this week’s New England Journal of Medicine, a team of U.K. and U.S. researchers, partly supported by NIH, examined the use of ACE inhibitors and other angiotensin-receptor blockers (ARBs) in people with COVID-19. The team, led by Scott D. Solomon of Brigham and Women’s Hospital and Harvard Medical School, Boston, found that current evidence in humans is insufficient to support or refute claims that ACE inhibitors or ARBs may be helpful or harmful to individuals with COVID-19.
The researchers concluded that these anti-hypertensive drugs should be continued in people who have or at-risk for COVID-19, stating: “Although additional data may further inform the treatment of high-risk patients … clinicians need to be cognizant of the unintended consequences of prematurely discontinuing proven therapies in response to hypothetical concerns.” [2]
Research is underway to generate needed data on the use of ACE inhibitors and similar drugs in the context of the COVID-19 pandemic, as well as to understand more about the basic mechanisms underlying this rapidly spreading viral disease. This kind of fundamental research isn’t necessarily the stuff that will make headlines, but it likely will prove vital to guiding the design of effective drugs that can help bring this serious global health crisis under control.
References:
[1] Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Science. 27 March 2020. [Epub ahead of publication]
[2] Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. Vaduganathan M, Vardeny O, Michel T, McMurray J, Pfeffer MA, Solomon SD. 30 NEJM. March 2020 [Epub ahead of Publication]
Links:
Coronavirus (COVID-19) (NIH)
COVID-19, MERS & SARS (National Institute of Allergy and Infectious Diseases/NIH)
Transformative High Resolution Cryo-Electron Microscopy (Common Fund/NIH)
Qiang Zhou (Westlake Institute for Advanced Study, Zhejiang Province)
Scott D. Solomon (Brigham and Women’s Hospital, Boston)
NIH Support: National Center for Advancing Translational Sciences; National Heart, Lung, and Blood Institute
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: ACE inhibitors, ACE2, angiotensin converting enzyme 2, angiotensin-receptor blockers, ARB, basic research, blood pressure, China, coronavirus, COVID-19, cryo-EM, drug design, drug development, heart, heart disease, high blood pressure, high-resolution imaging, hypertension, imaging, infectious disease, lungs, novel coronavirus, pandemic, protein receptor, SARS-CoV-2, spike protein, structural biology, targeted therapy, viral pandemics, virology, virus
Genomic Study Points to Natural Origin of COVID-19
Posted on by Dr. Francis Collins

No matter where you go online these days, there’s bound to be discussion of coronavirus disease 2019 (COVID-19). Some folks are even making outrageous claims that the new coronavirus causing the pandemic was engineered in a lab and deliberately released to make people sick. A new study debunks such claims by providing scientific evidence that this novel coronavirus arose naturally.
The reassuring findings are the result of genomic analyses conducted by an international research team, partly supported by NIH. In their study in the journal Nature Medicine, Kristian Andersen, Scripps Research Institute, La Jolla, CA; Robert Garry, Tulane University School of Medicine, New Orleans; and their colleagues used sophisticated bioinformatic tools to compare publicly available genomic data from several coronaviruses, including the new one that causes COVID-19.
The researchers began by homing in on the parts of the coronavirus genomes that encode the spike proteins that give this family of viruses their distinctive crown-like appearance. (By the way, “corona” is Latin for “crown.”) All coronaviruses rely on spike proteins to infect other cells. But, over time, each coronavirus has fashioned these proteins a little differently, and the evolutionary clues about these modifications are spelled out in their genomes.
The genomic data of the new coronavirus responsible for COVID-19 show that its spike protein contains some unique adaptations. One of these adaptations provides special ability of this coronavirus to bind to a specific protein on human cells called angiotensin converting enzyme (ACE2). A related coronavirus that causes severe acute respiratory syndrome (SARS) in humans also seeks out ACE2.
Existing computer models predicted that the new coronavirus would not bind to ACE2 as well as the SARS virus. However, to their surprise, the researchers found that the spike protein of the new coronavirus actually bound far better than computer predictions, likely because of natural selection on ACE2 that enabled the virus to take advantage of a previously unidentified alternate binding site. Researchers said this provides strong evidence that that new virus was not the product of purposeful manipulation in a lab. In fact, any bioengineer trying to design a coronavirus that threatened human health probably would never have chosen this particular conformation for a spike protein.
The researchers went on to analyze genomic data related to the overall molecular structure, or backbone, of the new coronavirus. Their analysis showed that the backbone of the new coronavirus’s genome most closely resembles that of a bat coronavirus discovered after the COVID-19 pandemic began. However, the region that binds ACE2 resembles a novel virus found in pangolins, a strange-looking animal sometimes called a scaly anteater. This provides additional evidence that the coronavirus that causes COVID-19 almost certainly originated in nature. If the new coronavirus had been manufactured in a lab, scientists most likely would have used the backbones of coronaviruses already known to cause serious diseases in humans.
So, what is the natural origin of the novel coronavirus responsible for the COVID-19 pandemic? The researchers don’t yet have a precise answer. But they do offer two possible scenarios.
In the first scenario, as the new coronavirus evolved in its natural hosts, possibly bats or pangolins, its spike proteins mutated to bind to molecules similar in structure to the human ACE2 protein, thereby enabling it to infect human cells. This scenario seems to fit other recent outbreaks of coronavirus-caused disease in humans, such as SARS, which arose from cat-like civets; and Middle East respiratory syndrome (MERS), which arose from camels.
The second scenario is that the new coronavirus crossed from animals into humans before it became capable of causing human disease. Then, as a result of gradual evolutionary changes over years or perhaps decades, the virus eventually gained the ability to spread from human-to-human and cause serious, often life-threatening disease.
Either way, this study leaves little room to refute a natural origin for COVID-19. And that’s a good thing because it helps us keep focused on what really matters: observing good hygiene, practicing social distancing, and supporting the efforts of all the dedicated health-care professionals and researchers who are working so hard to address this major public health challenge.
Finally, next time you come across something about COVID-19 online that disturbs or puzzles you, I suggest going to FEMA’s new Coronavirus Rumor Control web site. It may not have all the answers to your questions, but it’s definitely a step in the right direction in helping to distinguish rumors from facts.
Reference:
[1] The proximal origin of SARS-CoV-2. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. Nat Med, 17 March 2020. [Epub ahead of publication]
Links:
Coronavirus (COVID-19) (NIH)
COVID-19, MERS & SARS (National Institute of Allergy and Infectious Diseases/NIH)
Andersen Lab (Scripps Research Institute, La Jolla, CA)
Robert Garry (Tulane University School of Medicine, New Orleans)
Coronavirus Rumor Control (FEMA)
NIH Support: National Institute of Allergy and Infectious Diseases; National Human Genome Research Institute
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: News
Tags: ACE2, bats, bioengineering, camels, civets, coronavirus, Coronavirus Rumor Control, COVID-19, evolutionary biology, FEMA, genomics, man-made, MERS, natural, natural origin, new coronavirus, pandemic, pangolin, SARS, SARS-CoV-2, social distancing, spike protein, viral pandemics, virology
Creative Minds: Preparing for Future Pandemics
Posted on by Dr. Francis Collins

Jonathan Abraham / Credit: ChieYu Lin
Growing up in Queens, NY, Jonathan Abraham developed a love for books and an interest in infectious diseases. One day Abraham got his hands on a copy of Laurie Garrett’s The Coming Plague, a 1990s bestseller warning of future global pandemics, and he sensed his life’s calling. He would help people around the world survive deadly viral outbreaks, particularly from Ebola, Marburg, and other really bad bugs that cause deadly hemorrhagic fevers.
Abraham, now a physician-scientist at Brigham and Women’s Hospital, Boston, continues to chase that dream. With support from an NIH Director’s 2016 Early Independence Award, Abraham has set out to help design the next generation of treatments to enable more people to survive future outbreaks of viral hemorrhagic fever. His research strategy: find antibodies in the blood of known survivors that helped them overcome their infections. With further study, he hopes to develop purified forms of the antibodies as potentially life-saving treatments for people whose own immune systems may not make them in time. This therapeutic strategy is called passive immunity.
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Tags: antibodies, cryo-electron microscopy, cryo-EM, Ebola, hemorrhagic fever, infectious diseases, junin virus, Marburg virus, neutralizing antibodies, New World hemorrhagic fever, NIH Director’s 2016 Early Independence Award, pandemic, passive immunity, viral hemorrhagic fever, viral pandemics, virology, virus, x-ray crystallography
