mRNA vaccines
How COVID-19 Immunity Holds Up Over Time
Posted on by Lawrence Tabak, D.D.S., Ph.D.
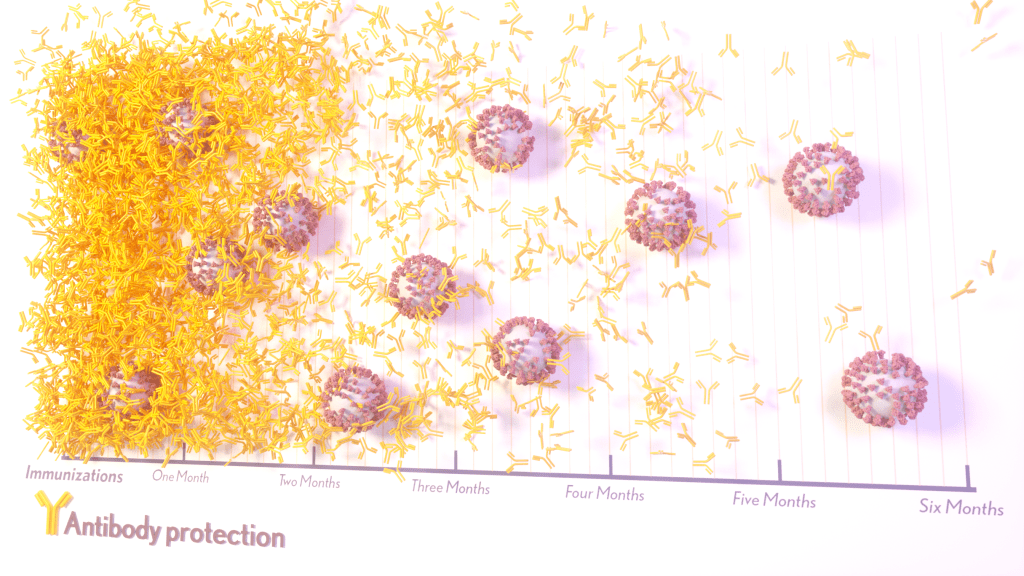
More than 215 million people in the United States are now fully vaccinated against the SARS-CoV-2 virus responsible for COVID-19 [1]. More than 40 percent—more than 94 million people—also have rolled up their sleeves for an additional, booster dose. Now, an NIH-funded study exploring how mRNA vaccines are performing over time comes as a reminder of just how important it will be to keep those COVID-19 vaccines up to date as coronavirus variants continue to circulate.
The results, published in the journal Science Translational Medicine, show that people who received two doses of either the Pfizer or Moderna COVID-19 mRNA vaccines did generate needed virus-neutralizing antibodies [2]. But levels of those antibodies dropped considerably after six months, suggesting declining immunity over time.
The data also reveal that study participants had much reduced protection against newer SARS-CoV-2 variants, including Delta and Omicron. While antibody protection remained stronger in people who’d also had a breakthrough infection, even that didn’t appear to offer much protection against infection by the Omicron variant.
The new study comes from a team led by Shan-Lu Liu at The Ohio State University, Columbus. They wanted to explore how well vaccine-acquired immune protection holds up over time, especially in light of newly arising SARS-CoV-2 variants.
This is an important issue going forward because mRNA vaccines train the immune system to produce antibodies against the spike proteins that crown the surface of the SARS-CoV-2 coronavirus. These new variants often have mutated, or slightly changed, spike proteins compared to the original one the immune system has been trained to detect, potentially dampening the immune response.
In the study, the team collected serum samples from 48 fully vaccinated health care workers at four key time points: 1) before vaccination, 2) three weeks after the first dose, 3) one month after the second dose, and 4) six months after the second dose.
They then tested the ability of antibodies in those samples to neutralize spike proteins as a correlate for how well a vaccine works to prevent infection. The spike proteins represented five major SARS-CoV-2 variants. The variants included D614G, which arose very soon after the coronavirus first was identified in Wuhan and quickly took over, as well as Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2), and Omicron (B.1.1.529).
The researchers explored in the lab how neutralizing antibodies within those serum samples reacted to SARS-CoV-2 pseudoviruses representing each of the five variants. SARS-CoV-2 pseudoviruses are harmless viruses engineered, in this case, to bear coronavirus spike proteins on their surfaces. Because they don’t replicate, they are safe to study without specially designed biosafety facilities.
At any of the four time points, antibodies showed a minimal ability to neutralize the Omicron spike protein, which harbors about 30 mutations. These findings are consistent with an earlier study showing a significant decline in neutralizing antibodies against Omicron in people who’ve received the initial series of two shots, with improved neutralizing ability following an additional booster dose.
The neutralizing ability of antibodies against all other spike variants showed a dramatic decline from 1 to 6 months after the second dose. While there was a marked decline over time after both vaccines, samples from health care workers who’d received the Moderna vaccine showed about twice the neutralizing ability of those who’d received the Pfizer vaccine. The data also suggests greater immune protection in fully vaccinated healthcare workers who’d had a breakthrough infection with SARS-CoV-2.
In addition to recommending full vaccination for all eligible individuals, the Centers for Disease Control and Prevention (CDC) now recommends everyone 12 years and up should get a booster dose of either the Pfizer or Moderna vaccines at least five months after completing the primary series of two shots [3]. Those who’ve received the Johnson & Johnson vaccine should get a booster at least two months after receiving the initial dose.
While plenty of questions about the durability of COVID-19 immunity over time remain, it’s clear that the rapid deployment of multiple vaccines over the course of this pandemic already has saved many lives and kept many more people out of the hospital. As the Omicron threat subsides and we start to look forward to better days ahead, it will remain critical for researchers and policymakers to continually evaluate and revise vaccination strategies and recommendations, to keep our defenses up as this virus continues to evolve.
References:
[1] COVID-19 vaccinations in the United States. Centers for Disease Control and Prevention. February 27, 2022.
[2] Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Evans JP, Zeng C, Carlin C, Lozanski G, Saif LJ, Oltz EM, Gumina RJ, Liu SL. Sci Transl Med. 2022 Feb 15:eabn8057.
[3] COVID-19 vaccine booster shots. Centers for Disease Control and Prevention. Feb 2, 2022.
Links:
COVID-19 Research (NIH)
Shan-Lu Liu (The Ohio State University, Columbus)
NIH Support: National Institute of Allergy and Infectious Diseases; National Cancer Institute; National Heart, Lung, and Blood Institute; Eunice Kennedy Shriver National Institute of Child Health and Human Development
Latest on Omicron Variant and COVID-19 Vaccine Protection
Posted on by Dr. Francis Collins
There’s been great concern about the new Omicron variant of SARS-CoV-2, the coronavirus that causes COVID-19. A major reason is Omicron has accumulated over 50 mutations, including about 30 in the spike protein, the part of the coronavirus that mRNA vaccines teach our immune systems to attack. All of these genetic changes raise the possibility that Omicron could cause breakthrough infections in people who’ve already received a Pfizer or Moderna mRNA vaccine.
So, what does the science show? The first data to emerge present somewhat encouraging results. While our existing mRNA vaccines still offer some protection against Omicron, there appears to be a significant decline in neutralizing antibodies against this variant in people who have received two shots of an mRNA vaccine.
However, initial results of studies conducted both in the lab and in the real world show that people who get a booster shot, or third dose of vaccine, may be better protected. Though these data are preliminary, they suggest that getting a booster will help protect people already vaccinated from breakthrough or possible severe infections with Omicron during the winter months.
Though Omicron was discovered in South Africa only last month, researchers have been working around the clock to learn more about this variant. Last week brought the first wave of scientific data on Omicron, including interesting work from a research team led by Alex Sigal, Africa Health Research Institute, Durban, South Africa [1].
In lab studies working with live Omicron virus, the researchers showed that this variant still relies on the ACE2 receptor to infect human lung cells. That’s really good news. It means that the therapeutic tools already developed, including vaccines, should generally remain useful for combatting this new variant.
Sigal and colleagues also tested the ability of antibodies in the plasma from 12 fully vaccinated individuals to neutralize Omicron. Six of the individuals had no history of COVID-19. The other six had been infected with the original variant in the first wave of infections in South Africa.
As expected, the samples showed very strong neutralization against the original SARS-CoV-2 variant. However, antibodies from people who’d been previously vaccinated with the two-dose Pfizer vaccine took a significant hit against Omicron, showing about a 40-fold decline in neutralizing ability.
This escape from immunity wasn’t complete. Indeed, blood samples from five individuals showed relatively good antibody levels against Omicron. All five had previously been infected with SARS-CoV-2 in addition to being vaccinated. These findings add to evidence on the value of full vaccination for protecting against reinfections in people who’ve had COVID-19 previously.
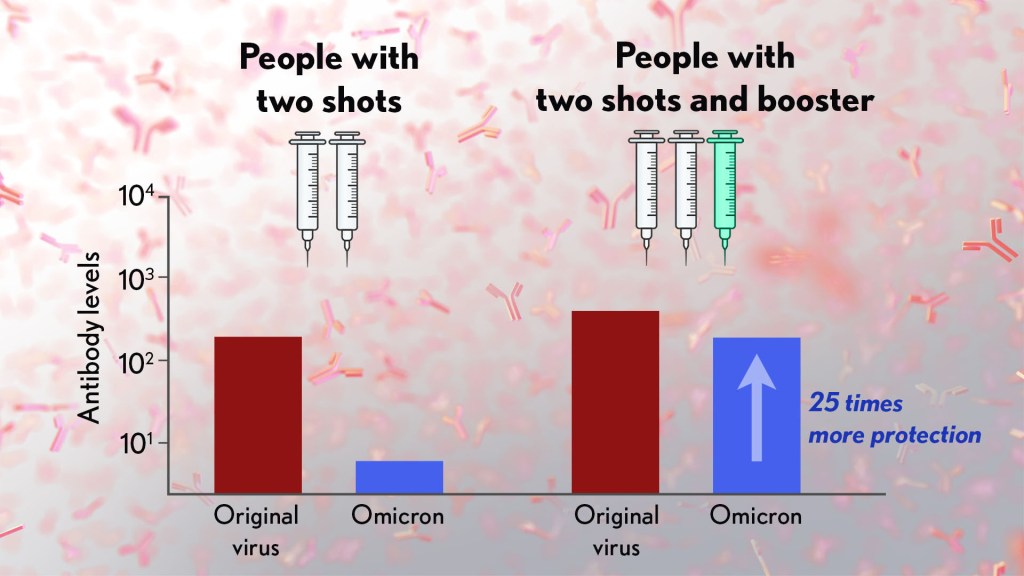
Also of great interest were the first results of the Pfizer study, which the company made available in a news release [2]. Pfizer researchers also conducted laboratory studies to test the neutralizing ability of blood samples from 19 individuals one month after a second shot compared to 20 others one month after a booster shot.
These studies showed that the neutralizing ability of samples from those who’d received two shots had a more than 25-fold decline relative to the original virus. Together with the South Africa data, it suggests that the two-dose series may not be enough to protect against breakthrough infections with the Omicron variant.
In much more encouraging news, their studies went on to show that a booster dose of the Pfizer vaccine raised antibody levels against Omicron to a level comparable to the two-dose regimen against the original variant (as shown in the figure above). While efforts already are underway to develop an Omicron-specific COVID-19 vaccine, these findings suggest that it’s already possible to get good protection against this new variant by getting a booster shot.
Very recently, real-world data from the United Kingdom, where Omicron cases are rising rapidly, are providing additional evidence for how boosters can help. In a preprint [3], Andrews et. al showed the effectiveness of two shots of Pfizer mRNA vaccine trended down after four months to about 40 percent. That’s not great, but note that 40 percent is far better than zero. So, clearly there is some protection provided.
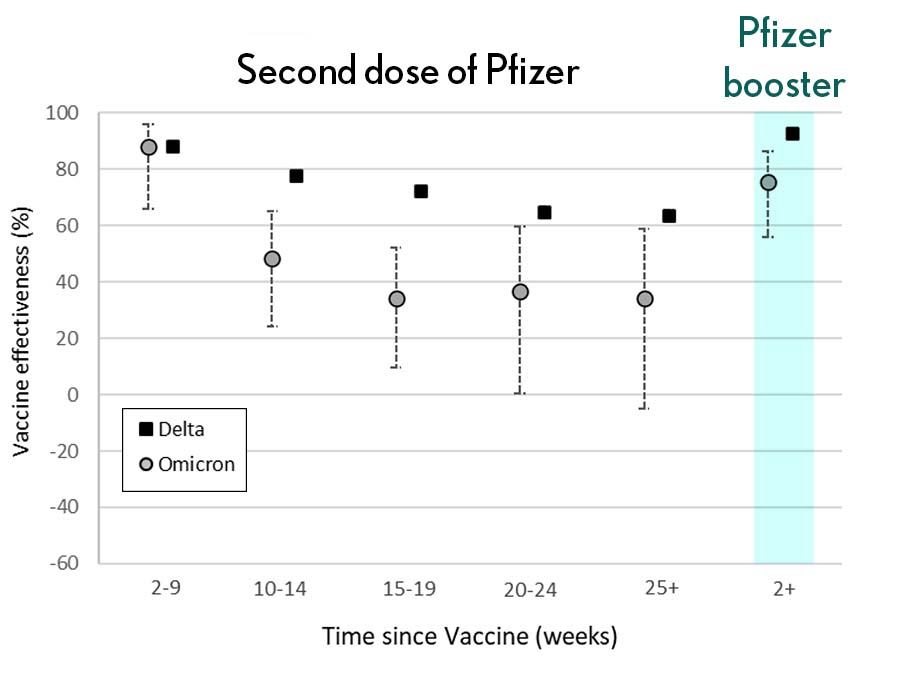
Most impressively (as shown in the figure from Andrews N, et al.) a booster substantially raised that vaccine effectiveness to about 80 percent. That’s not quite as high as for Delta, but certainly an encouraging result. Once again, these data show that boosting the immune system after a pause produces enhanced immunity against new viral variants, even though the booster was designed from the original virus. Your immune system is awfully clever. You get both quantitative and qualitative benefits.
It’s also worth noting that the Omicron variant mostly doesn’t have mutations in portions of its genome that are the targets of other aspects of vaccine-induced immunity, including T cells. These cells are part of the body’s second line of defense and are generally harder for viruses to escape. While T cells can’t prevent infection, they help protect against more severe illness and death.
It’s important to note that scientists around the world are also closely monitoring Omicron’s severity While this variant appears to be highly transmissible, and it is still early for rigorous conclusions, the initial research indicates this variant may actually produce milder illness than Delta, which is currently the dominant strain in the United States.
But there’s still a tremendous amount of research to be done that could change how we view Omicron. This research will take time and patience.
What won’t change, though, is that vaccines are the best way to protect yourself and others against COVID-19. (And these recent data provide an even-stronger reason to get a booster now if you are eligible.) Wearing a mask, especially in public indoor settings, offers good protection against the spread of all SARS-CoV-2 variants. If you’ve got symptoms or think you may have been exposed, get tested and stay home if you get a positive result. As we await more answers, it’s as important as ever to use all the tools available to keep yourself, your loved ones, and your community happy and healthy this holiday season.
References:
[1] SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. Sandile C, et al. Sandile C, et al. medRxiv preprint. December 9, 2021.
[2] Pfizer and BioNTech provide update on Omicron variant. Pfizer. December 8, 2021.
[3] Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. Andrews N, et al. KHub.net preprint. December 10, 2021.
Links:
COVID-19 Research (NIH)
Sigal Lab (Africa Health Research Institute, Durban, South Africa)
Teaching the Immune System to Attack Cancer with Greater Precision
Posted on by Dr. Francis Collins

To protect humans from COVID-19, the Pfizer and Moderna mRNA vaccines program human cells to translate the injected synthetic messenger RNA into the coronavirus spike protein, which then primes the immune system to arm itself against future appearances of that protein. It turns out that the immune system can also be trained to spot and attack distinctive proteins on cancer cells, killing them and leaving healthy cells potentially untouched.
While these precision cancer vaccines remain experimental, researchers continue to make basic discoveries that move the field forward. That includes a recent NIH-funded study in mice that helps to refine the selection of protein targets on tumors as a way to boost the immune response [1]. To enable this boost, the researchers first had to discover a possible solution to a longstanding challenge in developing precision cancer vaccines: T cell exhaustion.
The term refers to the immune system’s complement of T cells and their capacity to learn to recognize foreign proteins, also known as neoantigens, and attack them on cancer cells to shrink tumors. But these responding T cells can exhaust themselves attacking tumors, limiting the immune response and making its benefits short-lived.
In this latest study, published in the journal Cell, Tyler Jacks and Megan Burger, Massachusetts Institute of Technology, Cambridge, help to explain this phenomenon of T cell exhaustion. The researchers found in mice with lung tumors that the immune system initially responds as it should. It produces lots of T cells that target many different cancer-specific proteins.
Yet there’s a problem: various subsets of T cells get in each other’s way. They compete until, eventually, one of those subsets becomes the dominant T cell type. Even when those dominant T cells grow exhausted, they still remain in the tumor and keep out other T cells, which might otherwise attack different neoantigens in the cancer.
Building on this basic discovery, the researchers came up with a strategy for developing cancer vaccines that can “awaken” T cells and reinvigorate the body’s natural cancer-fighting abilities. The strategy might seem counterintuitive. The researchers vaccinated mice with neoantigens that provide a weak but encouraging signal to the immune cells responsible for presenting the distinctive cancer protein target, or antigen, to T cells. It’s those T cells that tend to get suppressed in competition with other T cells.
When the researchers vaccinated the mice with one of those neoantigens, the otherwise suppressed T cells grew in numbers and better targeted the tumor. What’s more, the tumors shrank by more than 25 percent on average.
Research on this new strategy remains in its early stages. The researchers hope to learn if this approach to cancer vaccines might work even better when used in combination with immunotherapy drugs, which unleash the immune system against cancer in other ways.
It’s also possible that the recent and revolutionary success of mRNA vaccines for preventing COVID-19 actually could help. An important advantage of mRNA is that it’s easy for researchers to synthesize once they know the specific nucleic acid sequence of a protein target, and they can even combine different mRNA sequences to make a multiplex vaccine that primes the immune system to recognize multiple neoantigens. Now that we’ve seen how well mRNA vaccines work to prompt a desired immune response against COVID-19, this same technology can be used to speed the development and testing of future vaccines, including those designed precisely to fight cancer.
Reference:
[1] Antigen dominance hierarchies shape TCF1+ progenitor CD8 T cell phenotypes in tumors. Burger ML, Cruz AM, Crossland GE, Gaglia G, Ritch CC, Blatt SE, Bhutkar A, Canner D, Kienka T, Tavana SZ, Barandiaran AL, Garmilla A, Schenkel JM, Hillman M, de Los Rios Kobara I, Li A, Jaeger AM, Hwang WL, Westcott PMK, Manos MP, Holovatska MM, Hodi FS, Regev A, Santagata S, Jacks T. Cell. 2021 Sep 16;184(19):4996-5014.e26.
Links:
Cancer Treatment Vaccines (National Cancer Institute/NIH)
The Jacks Lab (Massachusetts Institute of Technology, Cambridge)
NIH Support: National Cancer Institute; National Heart, Lung, and Blood Institute
