126 Search Results for "pain"
Machine Learning Study Offers Clues to Why Some People Have Rheumatoid Arthritis Pain Without Inflammation
Posted on by Dr. Monica M. Bertagnolli

About 1.5 million adults in the U.S. are living with rheumatoid arthritis (RA), an autoimmune disease in which the immune system attacks joint tissue, causing inflammation, swelling, and pain. Treatments often do a good job fighting inflammation to slow or even stop joint damage and ease pain. But this doesn’t work for everyone. Many people with RA don’t find pain relief, even with the strongest anti-inflammatory, disease-modifying therapies now available.
Why is that? A new study supported in part by NIH and reported in Science Translational Medicine has an intriguing answer.1 The findings suggest that in some people with RA, the joint lining may direct the growth of pain-sensing neurons to cause pain in the absence of inflammation. This discovery, made possible with the help of machine learning, suggests potential new ways to treat this painful disease.
The findings come from a team led by Fei Wang, Weill Cornell Medicine, New York City, and Dana E. Orange, Rockefeller University, New York City. They were inspired by recent studies showing that RA pain and inflammation don’t always go together. In fact, people with RA who have limited inflammation in some cases report just as much pain as those who have extreme inflammation. As a result, they also tend to get less benefit from anti-inflammatory drugs.
To find out why, the researchers studied the soft tissue, or synovium, lining the spaces of the joints from people with this less common form of RA. They were in search of underlying differences in gene activity to explain the pain without inflammation. They knew it wouldn’t be easy, given the variation in the way people experience and report pain and the limited availability of surgically removed tissue samples. To overcome those roadblocks, they developed a machine learning approach that could pinpoint pain-associated patterns of gene activity in the complex data that would otherwise be too difficult to discern.
Their RNA sequencing analysis turned up 815 genes that were expressed at unusually high levels in the joint tissue of 22 people who had RA pain with low inflammation. They also confirmed this same pattern of gene activity in a second group of patients with early untreated RA and little inflammation.
The researchers went on to find that this pattern was clearest in fibroblast cells (a major cell type of the synovium) which provide the structural framework of the joint space, but become a key driver of inflammation and joint damage in RA. Those fibroblasts also expressed a gene that encodes a protein called netrin-4, which is related to a family of proteins that play a role in the growth of neurons. It led them to wonder whether the joint tissue might be producing substances that could alter pain-sensing nerves to cause pain.
To learn more, they turned to studies in mice. They found that fluid collected from joint fibroblast cell cultures and netrin-4 made mouse neurons sprout new branches carrying pain receptors in the lab. The findings suggested that the RA joint lining was indeed producing substances that could lead to the growth of pain-sensing neurons.
To see if this might play a role in people with RA and little inflammation, they looked closely at the joints. Those images revealed an abundance of blood vessels that could nurture tissue growth. Those vessels were also surrounded by pain-sensing nerve fibers extending toward the joint lining in places where there was an abnormal amount of tissue growth.
The researchers think this process explains why painful, arthritic joints sometimes feel squishy and swollen even when they aren’t inflamed. In future studies, they want to learn more about which sensory neurons are specifically affected, noting that there are about a dozen different types. While much more study is needed, their goal is to find promising new ways to treat RA by targeting this underlying process, giving more people with RA much needed pain relief.
Reference:
[1] Bai Z, et al. Synovial fibroblast gene expression is associated with sensory nerve growth and pain in rheumatoid arthritis. Science Translational Medicine. DOI: 10.1126/scitranslmed.adk3506 (2024).
NIH Support: National Institute of Arthritis and Musculoskeletal and Skin Diseases
Pain Circuit Discovery in the Brain Suggests Promising Alternative to Opioid Painkillers
Posted on by Lawrence Tabak, D.D.S., Ph.D.
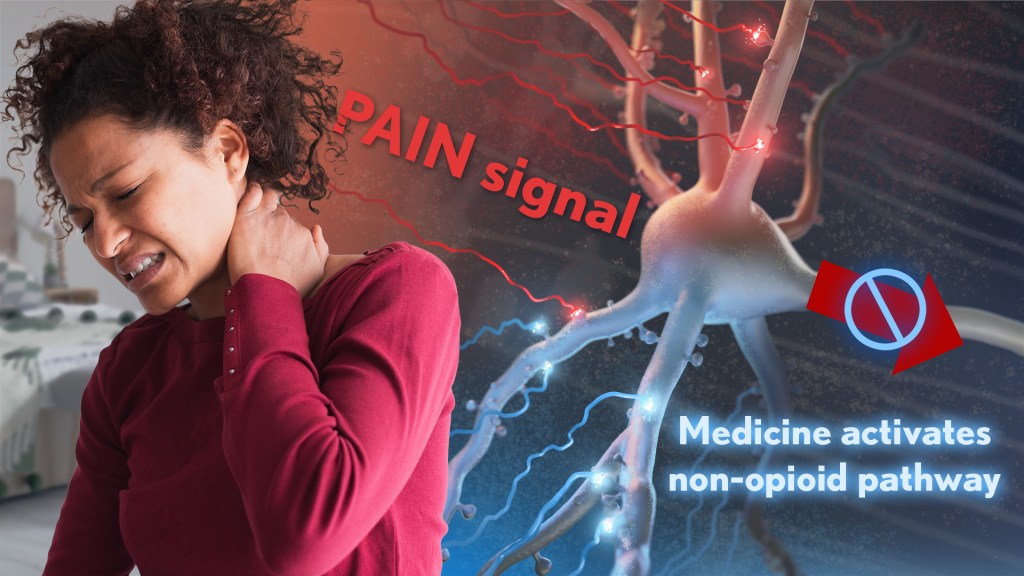
Chronic pain is an often-debilitating health condition and serious public health concern, affecting more than 50 million Americans.1 The opioid and overdose crisis, which stems from inadequate pain treatment, continues to have a devastating impact on families and communities across the country. To combat both challenges, we urgently need new ways to treat acute and chronic pain effectively without the many downsides of opioids.
While there are already multiple classes of non-opioid pain medications and other approaches to manage pain, unfortunately none have proved as effective as opioids when it comes to pain relief. So, I’m encouraged to see that an NIH-funded team now has preclinical evidence of a promising alternative target for pain-relieving medicines in the brain.2
Rather than activating opioid receptors, the new approach targets receptors for a nerve messenger known as acetylcholine in a portion of the brain involved in pain control. Based on findings from animal models, it appears that treatments targeting acetylcholine could offer pain relief even in people who have reduced responsiveness to opioids. Their findings suggest that the treatment approach has the potential to remain effective in combatting pain long-term and with limited risk for withdrawal symptoms or addiction.
The researchers, led by Daniel McGehee, University of Chicago, focused their attention on non-opioid pathways in the ventrolateral periaqueductal gray (vlPAG), a brain area involved in pain control. They had previously shown that activating acetylcholine receptors, which are part of the vlPAG’s underlying circuitry, could relieve pain.3 However, they found that when the body is experiencing pain, it unexpectedly suppresses acetylcholine rather than releasing more.
To understand how and why this is happening, McGehee and Shivang Sullere, now a postdoctoral fellow at Harvard Medical School, conducted studies in mice to understand how acetylcholine is released under various pain states. They found that mice treated with a drug that stimulates an acetylcholine receptor known as alpha-7 (⍺7) initially led to more activity in the nervous system. But this activity quickly gave way to a prolonged inactive or quiet state that delivered pain relief. Interestingly, this unexpected inhibitory effect lasted for several hours.
Additional studies in mice that had developed a tolerance to opioids showed the same long-lasting pain relief. This encouraging finding was expected since opioids use a pathway separate from acetylcholine. In more good news, the animals didn’t show any signs of dependence or addiction either. For instance, in the absence of pain, they didn’t prefer spending time in environments where they’d receive the ⍺7-targeted drug.
Imaging studies measuring brain activity revealed greater activity in cells expressing ⍺7 with higher levels of pain. When that activity was blocked, pain levels dropped. The finding suggests to the researchers it may be possible to monitor pain levels through brain imaging. It’s also possible the acetylcholine circuitry in the brain may play a role in the process whereby acute or temporary pain becomes chronic.
Finding treatments to modify acetylcholine levels or target acetylcholine receptors may therefore offer a means to treat pain and prevent it from becoming chronic. Encouragingly, drugs acting on these receptors already have been tested for use in people for treating other health conditions. It will now be important to learn whether these existing therapeutics or others like them may act as highly effective, non-addictive painkillers, with important implications for alleviating chronic pain.
References:
[1] NIH HEAL Initiative Research Plan. NIH HEAL Initiative.
[2] Sullere S et al. A cholinergic circuit that relieves pain despite opioid tolerance. Neuron. DOI: 10.1016/j.neuron.2023.08.017 (2023).
[3] Umana IC et al. Nicotinic modulation of descending pain control circuitry. Pain. PMID: 28817416; PMCID: PMC5873975 (2017).
Links:
The Helping to End Addiction Long-term® (HEAL) Initiative (NIH)
Pain (National Institute of Neurological Disorders and Stroke/NIH)
Opioids (National Institute on Drug Abuse/NIH)
Daniel McGehee (University of Chicago, Illinois)
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute on Drug Abuse
A Whole Person Approach to Lifting the Burden of Chronic Pain Among Service Members and Veterans
Posted on by Helene M. Langevin, M.D., National Center for Complementary and Integrative Health

Chronic pain and its companion crisis of opioid misuse have taken a terrible toll on Americans. But the impact has been even greater on U.S. service members and veterans, who often deal with the compounded factors of service-related injuries and traumatic stress.
For example, among soldiers in a leading U.S. Army unit, 44 percent had chronic pain and 15 percent used opioids after a combat deployment. That compares to 26 percent and 4 percent, respectively, in the general population [1,2].
This disproportionate burden of chronic pain among veterans [3] and service members led NIH’s National Center for Complementary and Integrative Health (NCCIH) to act. We forged a collaboration in 2017 across NIH, U.S. Department of Defense (DOD), and U.S. Department of Veteran’s Affairs (VA) to establish the Pain Management Collaboratory (PMC).
The PMC’s research focusing on the implementation and evaluation of nondrug approaches for the management of pain is urgently needed in the military and across our entire country. Nondrug approaches require a shift in thinking. Rather than focusing solely on blocking pain temporarily using analgesics, nondrug approaches work with the mind and body to promote the resolution of chronic pain and the long-term restoration of health through techniques and practices such as manual therapy, yoga, and mindfulness-based interventions.
Addressing chronic pain in ways that don’t only rely on drugs means addressing underlying issues, such as joints and connective tissue that lack adequate movement or training our brains to “turn down the volume” on pain signals. Using mind and body practices to reduce pain can help promote health in other ways. Possible “fringe benefits” include better sleep, more energy for physical activity, a better mindset for making good nutritional choices, and/or improved mood.
Indeed, there is a growing body of research on the benefits of nondrug approaches to address chronic pain. What is so powerful about PMC is it puts this knowledge to work by embedding research within military health care settings.
The PMC supports a shared resource center and 11 large-scale pragmatic clinical trials. Within this real-world health care setting, the clinical trials have enrolled more than 8,200 participants across 42 veteran and military health systems. These studies offer both strength in numbers and insights into what happens when learnings from controlled clinical trials collide with the realities of health care delivery and the complexities of daily life. [4]
Central to the PMC partnership is whole person health. Too often, we see health through the prism of separate parts—for example, a person’s cardiovascular, digestive, and mental health problems are viewed as co-occurring rather than as interrelated conditions. A whole person framework—a central focus of NCCIH’s current Strategic Plan—brings the parts back together and recognizes that health exists across multiple interconnected body systems and domains: biological, behavioral, social, and environmental.
The VA’s implementation of a whole health model [5] and their unique closed-loop health care system offers an opportunity to deliver care, conduct research, and illustrate what happens when people receive coordinated care that treats the whole person. In fact, VA’s leadership in this area was the impetus for a recent report by the National Academies of Sciences, Engineering, and Medicine. The report underscored the importance of implementing whole person health care in all settings and for every American.
There are many opportunities ahead for this interagency collaboration. It will help to achieve an important shift, from treating problems one at a time to promoting overall military readiness, resilience, and well-being for U.S. service members and veterans.
Congress appropriated $5 million to NCCIH in fiscal year 2023 to enhance pain research with a special emphasis on military populations. These additional resources will allow NCCIH to support more complex studies in understanding how multiple therapeutic approaches that impact multiple body systems can impact chronic pain.
Meanwhile, programs like the DOD’s Consortium for Health and Military Performance (CHAMP) will continue to translate these lessons learned into accessible pain management information that service members can use in promoting and maintaining their health.
While the PMC’s research program specifically targets the military community, this growing body of knowledge will benefit us all. Understanding how to better manage chronic pain and offering more treatment options for those who want to avoid the risks of opioids will help us all build resilience and restore health of the whole person.
References:
[1] Chronic pain and opioid use in US soldiers after combat deployment. Toblin RL, Quartana PJ, Riviere LA, Walper KC, Hoge CW. JAMA Intern. Med. 2014 Aug;174(8):1400-1401.
[2] Pain and opioids in the military: We must do better. Jonas WB, Schoomaker EB. JAMA Intern. Med. 2014 Aug;174(8):1402-1403
[3] Severe pain in veterans: The effect of age and sex, and comparisons with the general population. Nahin RL. J Pain. 2017 Mar; 18(3):247-254.
[4] Justice and equity in pragmatic clinical trials: Considerations for pain research within integrated health systems. Ali J, Davis AF, Burgess DJ, Rhon DI, Vining R, Young-McCaughan S, Green S, Kerns RD. Learn Health Sys. 2021 Oct 19;6(2): e10291
[5] The APPROACH trial: Assessing pain, patient-reported outcomes, and complementary and integrative health. Zeliadt S, Coggeshall S, Thomas E, Gelman H, Taylor S. Clin. Trials. 2020 Aug;17(4):351-359.
Links:
National Center for Complementary and Integrative Health (NIH)
NCCIH Strategic Plan FY 2021-2025: Mapping a Pathway to Research on Whole Person Health (NIH)
Pain Management Collaboratory (Yale University, New Haven, CT)
Whole Health (U.S Department of Veteran’s Affairs, Washington, D.C.)
Consortium for Health and Military Performance (Department of Defense, Bethesda, MD)
Achieving Whole Health: A New Approach for Veterans and the Nation. (National Academies of Sciences, Engineering, and Medicine, Washington, D.C.)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 26th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Could CRISPR Gene-Editing Technology Be an Answer to Chronic Pain?
Posted on by Dr. Francis Collins

Gene editing has shown great promise as a non-heritable way to treat a wide range of conditions, including many genetic diseases and more recently, even COVID-19. But could a version of the CRISPR gene-editing tool also help deliver long-lasting pain relief without the risk of addiction associated with prescription opioid drugs?
In work recently published in the journal Science Translational Medicine, researchers demonstrated in mice that a modified version of the CRISPR system can be used to “turn off” a gene in critical neurons to block the transmission of pain signals [1]. While much more study is needed and the approach is still far from being tested in people, the findings suggest that this new CRISPR-based strategy could form the basis for a whole new way to manage chronic pain.
This novel approach to treating chronic pain occurred to Ana Moreno, the study’s first author, when she was a Ph.D. student in the NIH-supported lab of Prashant Mali, University of California, San Diego. Mali had been studying a wide range of novel gene- and cell-based therapeutics. While reading up on both, Moreno landed on a paper about a mutation in a gene that encodes a pain-enhancing protein in spinal neurons called NaV1.7.
Moreno read that kids born with a loss-of-function mutation in this gene have a rare condition known as congenital insensitivity to pain (CIP). They literally don’t sense and respond to pain. Although these children often fail to recognize serious injuries because of the absence of pain to alert them, they have no other noticeable physical effects of the condition.
For Moreno, something clicked. What if it were possible to engineer a new kind of treatment—one designed to turn this gene down or fully off and stop people from feeling chronic pain?
Moreno also had an idea about how to do it. She’d been working on repressing or “turning off” genes using a version of CRISPR known as “dead” Cas9 [2]. In CRISPR systems designed to edit DNA, the Cas9 enzyme is often likened to a pair of scissors. Its job is to cut DNA in just the right spot with the help of an RNA guide. However, CRISPR-dead Cas9 no longer has any ability to cut DNA. It simply sticks to its gene target and blocks its expression. Another advantage is that the system won’t lead to any permanent DNA changes, since any treatment based on CRISPR-dead Cas9 might be safely reversed.
After establishing that the technique worked in cells, Moreno and colleagues moved to studies of laboratory mice. They injected viral vectors carrying the CRISPR treatment into mice with different types of chronic pain, including inflammatory and chemotherapy-induced pain.
Moreno and colleagues determined that all the mice showed evidence of durable pain relief. Remarkably, the treatment also lasted for three months or more and, importantly, without any signs of side effects. The researchers are also exploring another approach to do the same thing using a different set of editing tools called zinc finger nucleases (ZFNs).
The researchers say that one of these approaches might one day work for people with a large number of chronic pain conditions that involve transmission of the pain signal through NaV1.7. That includes diabetic polyneuropathy, sciatica, and osteoarthritis. It also could provide relief for patients undergoing chemotherapy, along with those suffering from many other conditions. Moreno and Mali have co-founded the spinoff company Navega Therapeutics, San Diego, CA, to work on the preclinical steps necessary to help move their approach closer to the clinic.
Chronic pain is a devastating public health problem. While opioids are effective for acute pain, they can do more harm than good for many chronic pain conditions, and they are responsible for a nationwide crisis of addiction and drug overdose deaths [3]. We cannot solve any of these problems without finding new ways to treat chronic pain. As we look to the future, it’s hopeful that innovative new therapeutics such as this gene-editing system could one day help to bring much needed relief.
References:
[1] Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Moreno AM, Alemán F, Catroli GF, Hunt M, Hu M, Dailamy A, Pla A, Woller SA, Palmer N, Parekh U, McDonald D, Roberts AJ, Goodwill V, Dryden I, Hevner RF, Delay L, Gonçalves Dos Santos G, Yaksh TL, Mali P. Sci Transl Med. 2021 Mar 10;13(584):eaay9056.
[2] Nuclease dead Cas9 is a programmable roadblock for DNA replication. Whinn KS, Kaur G, Lewis JS, Schauer GD, Mueller SH, Jergic S, Maynard H, Gan ZY, Naganbabu M, Bruchez MP, O’Donnell ME, Dixon NE, van Oijen AM, Ghodke H. Sci Rep. 2019 Sep 16;9(1):13292.
[3] Drug Overdose Deaths. Centers for Disease Control and Prevention.
Links:
Congenital insensitivity to pain (National Center for Advancing Translational Sciences/NIH)
Opioids (National Institute on Drug Abuse/NIH)
Mali Lab (University of California, San Diego)
Navega Therapeutics (San Diego, CA)
NIH Support: National Human Genome Research Institute; National Cancer Institute; National Institute of General Medical Sciences; National Institute of Neurological Disorders and Stroke
#PainMonth18 Twitter Chat
Posted on by Dr. Francis Collins

A look behind the scenes at the #PainMonth18 Twitter Chat. I’m sitting with Alex Azar, secretary of Health and Human Services (HHS), and we’re watching a brief video. The twitter chat took place on September 18 in Washington, D.C. in recognition of Pain Awareness Month. Credit: HHS
Researchers Elucidate Role of Stress Gene in Chronic Pain
Posted on by Dr. Francis Collins

Credit: Getty Images/simonkr
For most people, pain eventually fades away as an injury heals. But for others, the pain persists beyond the initial healing and becomes chronic, hanging on for weeks, months, or even years. Now, we may have uncovered an answer to help explain why: subtle differences in a gene that controls how the body responds to stress.
In a recent study of more than 1,600 people injured in traffic accidents, researchers discovered that individuals with a certain variant in a stress-controlling gene, called FKBP5, were more likely to develop chronic pain than those with other variants [1]. These findings may point to new non-addictive strategies for preventing or controlling chronic pain, and underscore the importance of NIH-funded research for tackling our nation’s opioid overuse crisis.
Managing Chronic Pain: Opioids Are Often Not the Answer
Posted on by Dr. Francis Collins
 The term “silent epidemic” sometimes gets overused in medicine. But, for prescription opioid drugs, the term fits disturbingly well. In 2012, more than 259 million prescriptions were written in the United States for Vicodin, OxyContin, and other opioid painkillers. That equals one bottle of pain pills for every U.S. adult. And here’s an even more distressing statistic: in 2011, overdoses of prescription painkillers, most unintentional, claimed the lives about 17,000 Americans—46 people a day [1].
The term “silent epidemic” sometimes gets overused in medicine. But, for prescription opioid drugs, the term fits disturbingly well. In 2012, more than 259 million prescriptions were written in the United States for Vicodin, OxyContin, and other opioid painkillers. That equals one bottle of pain pills for every U.S. adult. And here’s an even more distressing statistic: in 2011, overdoses of prescription painkillers, most unintentional, claimed the lives about 17,000 Americans—46 people a day [1].
The issue isn’t whether opioid painkillers have a role in managing chronic pain, such as that caused by cancer or severe injuries. They do. What’s been lacking is an unbiased review of the scientific literature to examine evidence on the safety of long-term prescription opioid use and the impact of such use on patients’ pain, function, and quality of life. The NIH Office of Disease Prevention (ODP) recently convened an independent panel to conduct such a review, and what it found is eye-opening. People with chronic pain have often been lumped into a single category and treated with generalized approaches, even though very little scientific evidence exists to support this practice.
How Does Acute Pain Become Chronic?
Posted on by Dr. Francis Collins
 Chronic pain is a major medical problem, affecting as many as 100 million Americans, robbing them of a full sense of well-being, disrupting their ability to work and earn a living, and causing untold suffering for the patient and family. This condition costs the country an estimated $560-635 billion annually—a staggering economic burden [1]. Worst of all, chronic pain is often resistant to treatment. NIH launched the Grand Challenge on Chronic Pain [2] to investigate how acute pain (which is part of daily experience) evolves into a chronic condition and what biological factors contribute to this transition.
Chronic pain is a major medical problem, affecting as many as 100 million Americans, robbing them of a full sense of well-being, disrupting their ability to work and earn a living, and causing untold suffering for the patient and family. This condition costs the country an estimated $560-635 billion annually—a staggering economic burden [1]. Worst of all, chronic pain is often resistant to treatment. NIH launched the Grand Challenge on Chronic Pain [2] to investigate how acute pain (which is part of daily experience) evolves into a chronic condition and what biological factors contribute to this transition.
But you may wonder: what, exactly, is the difference between acute and chronic pain?
What’s Behind that Morning Migraine? Community-Based Study Points to Differences in Perceived Sleep Quality, Energy on the Previous Day
Posted on by Dr. Monica M. Bertagnolli

Headaches are the most common form of pain and a major reason people miss work or school. Recurrent attacks of migraine headaches can be especially debilitating, involving moderate to severe throbbing and pulsating pain on one side of the head that sometimes lasts for days. Migraines and severe headaches affect about 1 in 5 women and about 1 in 10 men, making them one of the most prevalent of all neurological disorders.1 And yet there’s still a lot we don’t know about what causes headaches or how to predict when one is about to strike.
Now a new NIH-led study reported in the journal Neurology has some important insight.2 One of the things I especially appreciate about this new work is that it was conducted in a community setting rather than through a specialty clinic, with people tracking their own headache symptoms, sleep, mood, and more on a mobile phone app while they went about their daily lives. It means that the findings are extremely relevant to the average migraine sufferer who shows up in a primary care doctor’s office looking for help for their recurrent headaches.
The study, led by Kathleen Merikangas at NIH’s National Institute of Mental Health, Bethesda, MD, is part of a larger, community-based Family Study of Affective and Anxiety Spectrum Disorders. This ongoing study enrolls volunteers from the greater Washington, D.C., area with a range of disorders, including bipolar disorders, major depression, anxiety disorders, sleep disorders, and migraine along with their immediate family members. It also includes people with none of these disorders who serve as a control group. The goal is to learn more about the frequency of mood and other mental and physical disorders in families and how often they co-occur. This information can provide insight into the nature and causes for all these conditions.
While there will be much more to come from this ambitious work, the primary aim for this latest study was to look for links between a person’s perceived mood, sleep, energy, and stress and their likelihood for developing a headache. The study’s 477 participants, aged 7 to 84, included people with and without migraines who were also assessed for mood, anxiety, sleep disorders and other physical conditions. Women accounted for 291 of the study’s participants. Each were asked to track their emotional states, including anxiousness, mood, energy, stress, and headaches four times each day for two weeks. Each morning, they also reported on their sleep the night before.
The data showed that people with a morning migraine reported poorer quality sleep the night before. They also reported lower energy the day before. Interestingly, those factors didn’t lead to an increased risk of headaches in the afternoon or evening. Afternoon or evening headaches were more often preceded by higher stress levels or having higher-than-average energy the day before.
More specifically, people with poorer perceived sleep quality on average had a 22 percent greater chance for a headache attack the next morning. A decrease in the self-reported usual quality of sleep was also associated with an 18 percent increased chance of a headache the next morning. Similarly, a drop in the usual level of energy on the prior day was associated with a 16 percent greater chance of headache the next morning. In contrast, greater average levels of stress and substantially higher energy than usual the day before was associated with a 17 percent increased chance of headache later the next day.
Surprisingly, the study didn’t find any connection between feeling anxious or depressed with headaches on the next day after considering energy and sleep. However, Merikangas emphasizes that participants’ perceived differences in energy and sleep may not reflect objective measures of sleep patterns or energy, suggesting that the connection may still be based on changes in a person’s feelings about their underlying physical or emotional state in complex ways.
The findings suggest that changes in the body and brain are already taking place before a person first feels a headache, suggesting it may be possible to predict and prevent migraines or other headaches. It also adds to evidence for the usefulness of diaries or apps for headache sufferers to track their sleep, health, behavioral, and emotional states in real time to better understand and manage headache pain. Meanwhile, the researchers report that they’re continuing to explore other factors that may precede and trigger headaches, including dietary factors, changes in a person’s physiology such as stress hormone levels, and environmental factors, including weather, seasonal changes, and geography.
References:
[1] American Headache Society. The Prevalence of Migraine and Severe Headache.
[2] Lateef TM, et al. Association Between Electronic Diary-Rated Sleep, Mood, Energy, and Stress With Incident Headache in a Community-Based Sample. Neurology. DOI: 10.1212/WNL.0000000000208102. (2024).
NIH Support: National Institute of Mental Health
Next Page

