alpha-7
Pain Circuit Discovery in the Brain Suggests Promising Alternative to Opioid Painkillers
Posted on by Lawrence Tabak, D.D.S., Ph.D.
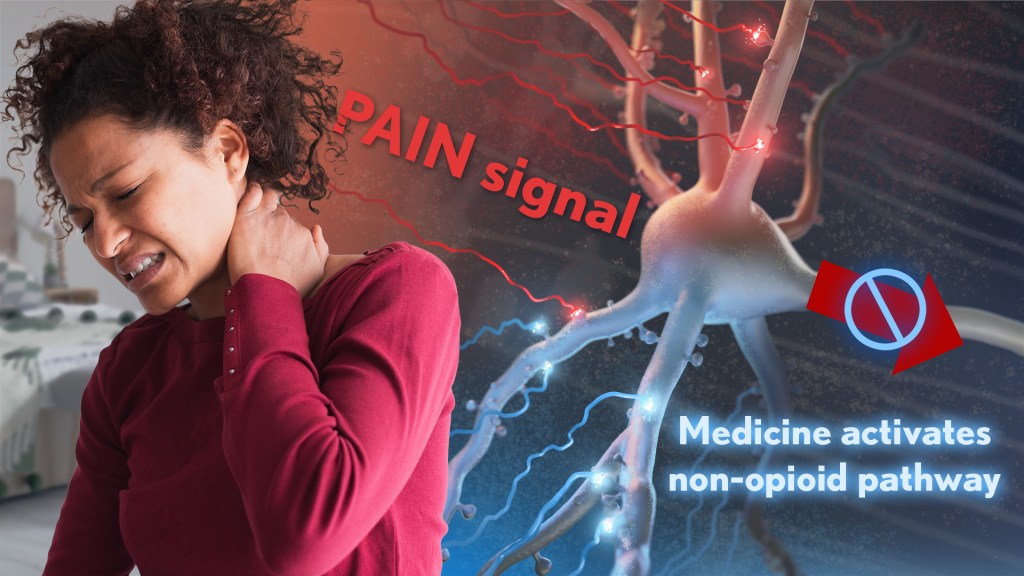
Chronic pain is an often-debilitating health condition and serious public health concern, affecting more than 50 million Americans.1 The opioid and overdose crisis, which stems from inadequate pain treatment, continues to have a devastating impact on families and communities across the country. To combat both challenges, we urgently need new ways to treat acute and chronic pain effectively without the many downsides of opioids.
While there are already multiple classes of non-opioid pain medications and other approaches to manage pain, unfortunately none have proved as effective as opioids when it comes to pain relief. So, I’m encouraged to see that an NIH-funded team now has preclinical evidence of a promising alternative target for pain-relieving medicines in the brain.2
Rather than activating opioid receptors, the new approach targets receptors for a nerve messenger known as acetylcholine in a portion of the brain involved in pain control. Based on findings from animal models, it appears that treatments targeting acetylcholine could offer pain relief even in people who have reduced responsiveness to opioids. Their findings suggest that the treatment approach has the potential to remain effective in combatting pain long-term and with limited risk for withdrawal symptoms or addiction.
The researchers, led by Daniel McGehee, University of Chicago, focused their attention on non-opioid pathways in the ventrolateral periaqueductal gray (vlPAG), a brain area involved in pain control. They had previously shown that activating acetylcholine receptors, which are part of the vlPAG’s underlying circuitry, could relieve pain.3 However, they found that when the body is experiencing pain, it unexpectedly suppresses acetylcholine rather than releasing more.
To understand how and why this is happening, McGehee and Shivang Sullere, now a postdoctoral fellow at Harvard Medical School, conducted studies in mice to understand how acetylcholine is released under various pain states. They found that mice treated with a drug that stimulates an acetylcholine receptor known as alpha-7 (⍺7) initially led to more activity in the nervous system. But this activity quickly gave way to a prolonged inactive or quiet state that delivered pain relief. Interestingly, this unexpected inhibitory effect lasted for several hours.
Additional studies in mice that had developed a tolerance to opioids showed the same long-lasting pain relief. This encouraging finding was expected since opioids use a pathway separate from acetylcholine. In more good news, the animals didn’t show any signs of dependence or addiction either. For instance, in the absence of pain, they didn’t prefer spending time in environments where they’d receive the ⍺7-targeted drug.
Imaging studies measuring brain activity revealed greater activity in cells expressing ⍺7 with higher levels of pain. When that activity was blocked, pain levels dropped. The finding suggests to the researchers it may be possible to monitor pain levels through brain imaging. It’s also possible the acetylcholine circuitry in the brain may play a role in the process whereby acute or temporary pain becomes chronic.
Finding treatments to modify acetylcholine levels or target acetylcholine receptors may therefore offer a means to treat pain and prevent it from becoming chronic. Encouragingly, drugs acting on these receptors already have been tested for use in people for treating other health conditions. It will now be important to learn whether these existing therapeutics or others like them may act as highly effective, non-addictive painkillers, with important implications for alleviating chronic pain.
References:
[1] NIH HEAL Initiative Research Plan. NIH HEAL Initiative.
[2] Sullere S et al. A cholinergic circuit that relieves pain despite opioid tolerance. Neuron. DOI: 10.1016/j.neuron.2023.08.017 (2023).
[3] Umana IC et al. Nicotinic modulation of descending pain control circuitry. Pain. PMID: 28817416; PMCID: PMC5873975 (2017).
Links:
The Helping to End Addiction Long-term® (HEAL) Initiative (NIH)
Pain (National Institute of Neurological Disorders and Stroke/NIH)
Opioids (National Institute on Drug Abuse/NIH)
Daniel McGehee (University of Chicago, Illinois)
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute on Drug Abuse
