125 Search Results for "pain"
How Neurons Make Connections
Posted on by Lawrence Tabak, D.D.S., Ph.D.

For many people, they are tiny pests. These fruit flies that sometimes hover over a bowl of peaches or a bunch of bananas. But for a dedicated community of researchers, fruit flies are an excellent model organism and source of information into how neurons self-organize during the insect’s early development and form a complex, fully functioning nervous system.
That’s the scientific story on display in this beautiful image of a larval fruit fly’s developing nervous system. Its subtext is: fundamental discoveries in the fruit fly, known in textbooks as Drosophila melanogaster, provide basic clues into the development and repair of the human nervous system. That’s because humans and fruit flies, though very distantly related through the millennia, still share many genes involved in their growth and development. In fact, 60 percent of the Drosophila genome is identical to ours.
Once hatched, as shown in this image, a larval fly uses neurons (magenta) to sense its environment. These include neurons that sense the way its body presses against the surrounding terrain, as needed to coordinate the movements of its segmented body parts and crawl in all directions.
This same set of neurons will generate painful sensations, such as the attack of a parasitic wasp. Paintbrush-like neurons in the fly’s developing head (magenta, left side) allow the insect to taste the sweetness of a peach or banana.
There is a second subtype of neurons, known as proprioceptors (green). These neurons will give the young fly its “sixth sense” understanding about where its body is positioned in space. The complete collection of developing neurons shown here are responsible for all the fly’s primary sensations. They also send these messages on to the insect’s central nervous system, which contains thousands of other neurons that are hidden from view.
Emily Heckman, now a postdoctoral researcher at the Michigan Neuroscience Institute, University of Michigan, Ann Arbor, captured this image during her graduate work in the lab of Chris Doe, University of Oregon, Eugene. For her keen eye, she received a trainee/early-career BioArt Award from the Federation of American Societies for Experimental Biology (FASEB), which each year celebrates the art of science.
The image is one of many from a much larger effort in the Doe lab that explores the way neurons that will partner find each other and link up to drive development. Heckman and Doe also wanted to know how neurons in the developing brain interconnect into integrated neural networks, or circuits, and respond when something goes wrong. To find out, they disrupted sensory neurons or forced them to take alternate paths and watched to see what would happen.
As published in the journal eLife [1], the system has an innate plasticity. Their findings show that developing sensory neurons instruct one another on how to meet up just right. If one suddenly takes an alternate route, its partner can still reach out and make the connection. Once an electrically active neural connection, or synapse, is made, the neural signals themselves slow or stop further growth. This kind of adaptation and crosstalk between neurons takes place only during a particular critical window during development.
Heckman says part of what she enjoys about the image is how it highlights that many sensory neurons develop simultaneously and in a coordinated process. What’s also great about visualizing these events in the fly embryo is that she and other researchers can track many individual neurons from the time they’re budding stem cells to when they become a fully functional and interconnected neural circuit.
So, the next time you see fruit flies hovering in the kitchen, just remember there’s more to their swarm than you think. Our lessons learned studying them will help point researchers toward new ways in people to restore or rebuild neural connections after devastating disruptions from injury or disease.
Reference:
Presynaptic contact and activity opposingly regulate postsynaptic dendrite outgrowth. Heckman EL, Doe CQ. Elife. 2022 Nov 30;11:e82093.
Links:
Research Organisms (National Institute of General Medical Sciences/NIH)
Doe Lab (University of Oregon, Eugene)
Emily Heckman (University of Michigan, Ann Arbor)
BioArt Awards (Federation of American Societies for Experimental Biology, Rockville, MD)
NIH Support: Eunice Kennedy Shriver National Institute of Child Health and Human Development
3D Animation Captures Viral Infection in Action
Posted on by Lawrence Tabak, D.D.S., Ph.D.
With the summer holiday season now in full swing, the blog will also swing into its annual August series. For most of the month, I will share with you just a small sampling of the colorful videos and snapshots of life captured in a select few of the hundreds of NIH-supported research labs around the country.
To get us started, let’s turn to the study of viruses. Researchers now can generate vast amounts of data relatively quickly on a virus of interest. But data are often displayed as numbers or two-dimensional digital images on a computer screen. For most virologists, it’s extremely helpful to see a virus and its data streaming in three dimensions. To do so, they turn to a technological tool that we all know so well: animation.
This research animation features the chikungunya virus, a sometimes debilitating, mosquito-borne pathogen transmitted mainly in developing countries in Africa, Asia and the Americas. The animation illustrates large amounts of research data to show how the chikungunya virus infects our cells and uses its specialized machinery to release its genetic material into the cell and seed future infections. Let’s take a look.
In the opening seconds, you see how receptor binding glycoproteins (light blue), which are proteins with a carbohydrate attached on the viral surface, dock with protein receptors (yellow) on a host cell. At five seconds, the virus is drawn inside the cell. The change in the color of the chikungunya particle shows that it’s coated in a vesicle, which helps the virus make its way unhindered through the cytoplasm.
At 10 seconds, the virus then enters an endosome, ubiquitous bubble-like compartments that transport material from outside the cell into the cytosol, the fluid part of the cytoplasm. Once inside the endosome, the acidic environment makes other glycoproteins (red, blue, yellow) on the viral surface change shape and become more flexible and dynamic. These glycoproteins serve as machinery that enables them to reach out and grab onto the surrounding endosome membrane, which ultimately will be fused with the virus’s own membrane.
As more of those fusion glycoproteins grab on, fold back on themselves, and form into hairpin-like shapes, they pull the membranes together. The animation illustrates not only the changes in protein organization, but the resulting effects on the integrity of the membrane structures as this dynamic process proceeds. At 53 seconds, the viral protein shell, or capsid (green), which contains the virus’ genetic instructions, is released back out into the cell where it will ultimately go on to make more virus.
This remarkable animation comes from Margot Riggi and Janet Iwasa, experts in visualizing biology at the University of Utah’s Animation Lab, Salt Lake City. Their data source was researcher Kelly Lee, University of Washington, Seattle, who collaborated closely with Riggi and Iwasa on this project. The final product was considered so outstanding that it took the top prize for short videos in the 2022 BioArt Awards competition, sponsored by the Federation of American Societies for Experimental Biology (FASEB).
The Lee lab uses various research methods to understand the specific shape-shifting changes that chikungunya and other viruses perform as they invade and infect cells. One of the lab’s key visual tools is cryo-electron microscopy (Cryo-EM), specifically cryo-electron tomography (cryo-ET). Cryto-ET enables complex 3D structures, including the intermediate state of biological reactions, to be captured and imaged in remarkably fine detail.
In a study in the journal Nature Communications [1] last year, Lee’s team used cryo-ET to reveal how the chikungunya virus invades and delivers its genetic cargo into human cells to initiate a new infection. While Lee’s cryo-ET data revealed stages of the virus entry process and fine structural details of changes to the virus as it enters a cell and starts an infection, it still represented a series of snapshots with missing steps in between. So, Lee’s lab teamed up with The Animation Lab to help beautifully fill in the gaps.
Visualizing chikungunya and similar viruses in action not only makes for informative animations, it helps researchers discover better potential targets to intervene in this process. This basic research continues to make progress, and so do ongoing efforts to develop a chikungunya vaccine [2] and specific treatments that would help give millions of people relief from the aches, pains, and rashes associated with this still-untreatable infection.
References:
[1] Visualization of conformational changes and membrane remodeling leading to genome delivery by viral class-II fusion machinery. Mangala Prasad V, Blijleven JS, Smit JM, Lee KK. Nat Commun. 2022 Aug 15;13(1):4772. doi: 10.1038/s41467-022-32431-9. PMID: 35970990; PMCID: PMC9378758.
[2] Experimental chikungunya vaccine is safe and well-tolerated in early trial, National Institute of Allergy and Infectious Diseases news release, April 27, 2020.
Links:
Chikungunya Virus (Centers for Disease Control and Prevention, Atlanta)
Global Arbovirus Initiative (World Health Organization, Geneva, Switzerland)
The Animation Lab (University of Utah, Salt Lake City)
Video: Janet Iwasa (TED Speaker)
Lee Lab (University of Washington, Seattle)
BioArt Awards (Federation of American Societies for Experimental Biology, Rockville, MD)
NIH Support: National Institute of General Medical Sciences; National Institute of Allergy and Infectious Diseases
Visit the New NIH Virtual Tour
Posted on by Lawrence Tabak, D.D.S., Ph.D.
Happy Fourth of July! Before everyone heads out to celebrate the holiday with their family and friends, I want to share this brief video with you. It’s an introduction to the brand-new NIH Virtual Tour that’s now available on our website. When time permits, I encourage everyone to take the full tour of our Bethesda, MD, main campus and explore this great institution of science, technological innovation, and, above all, hope.
Among the virtual tour’s many features is an interactive, aerial map of the 32 buildings on our Bethesda campus. By clicking on a highlighted building, you can explore an impressive multimedia gallery of photos, video clips, and other resources. The tour will allow you to learn more about NIH and the ways in which we help people live longer and healthier lives.
You also can learn more about NIH’s 27 Institutes and Centers, including the NIH Clinical Center and 20 other in-depth tour stops—from research labs to patient rooms—and hear directly from some of our impressive researchers, leaders, and patients. For example, you can learn about chronic pain research from a lab in the NIH Clinical Center or see the largest zebrafish facility in the world, housed in Building 6.
What I like most about the virtual tour is that it captures what makes NIH so special—the many amazing people who collaborate every day to discover ways to solve seemingly intractable research problems. I admire their commitment to follow the science wherever it may lead.
In fact, from its humble beginnings in a one-room laboratory in 1887, NIH has become the world’s largest funder of medical research, whether that’s mobilizing to combat a deadly pandemic or strategizing to help people with a rare disorder find answers.
Not only does NIH conduct groundbreaking research in its own labs and clinics, it also supports much of the medical research conducted at universities and institutions in your states and local communities. Whether in Bethesda or beyond the Beltway, this national research effort will continue to yield the needed understanding to turn discovery into better health, helping more people to flourish and lead fully productive lives, now and in the generations to come.
That’s certainly something we can all celebrate this holiday, the 247th birthday of our great nation that I’m so honored to serve. Have a great, but safe, Fourth of July, and I’ll see you back here soon to share another blog post and another story of NIH-supported research progress.
Links:
Virtual Tour (NIH)
Visitor Information (NIH)
Help for Babies Born Dependent on Opioids
Posted on by Lawrence Tabak, D.D.S., Ph.D.

It’s been estimated that every 18 minutes in the United States, a newborn baby starts life with painful withdrawals from exposure to opioids in the womb. It’s called neonatal opioid withdrawal syndrome (NOWS), and it makes for a challenging start in life. These infants may show an array of withdrawal symptoms, including tremors, extreme irritability, and problems eating and sleeping.
Many of these infants experience long, difficult hospital stays to help them manage their withdrawal symptoms. But because hospital staff have no established evidence-based treatment standards to rely on, there is substantial variation in NOWS treatment around the country. There also are many open questions about the safest and most-effective way to support these babies and their families.
But answers are coming. The New England Journal of Medicine just published clinical trial results that evaluated care for infants with NOWS and which offer some much needed—and rather encouraging—data for families and practitioners [1]. The data are from the Eating, Sleeping, Consoling for Neonatal Opioid Withdrawal (ESC-NOW) trial, led by Leslie W. Young, The University of Vermont’s Larner College of Medicine, Burlington, and her colleagues Lori Devlin and Stephanie Merhar.
The ESC-NOW study is supported through the Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW) Collaborative. ACT NOW is an essential part of the NIH Helping to End Addiction Long-term (HEAL) Initiative, an aggressive effort to speed scientific solutions to stem the national opioid public health crisis and improve lives.
The latest study puts to the test two different approaches to care for newborns with NOWS. The first approach relies on the Finnegan Neonatal Abstinence Scoring Tool. For almost 50 years, doctors primarily assessed NOWS using this tool. It is based on a scoring system of 21 signs of withdrawal, including disturbances in a baby’s nervous system, metabolism, breathing, digestion, and more. However, there have been concerns that this scoring tool has led to an overreliance on treating babies with opioid medications, including morphine and methadone.
The other approach is known as Eat, Sleep, Console (ESC) care [2]. First proposed in 2014, ESC care has been adopted in many hospitals around the world. Rather than focusing on a long list of physical signs of withdrawal, this approach relies on a simpler functional assessment of whether an infant can eat, sleep, and be consoled. It emphasizes treatments other than medication, such as skin-to-skin contact, breastfeeding, and care from their mothers or other caregivers in a calm and nurturing environment.
The ESC care approach places an emphasis on the use of supportive interventions and aims to empower families in the care and nurturing of their infants. While smaller quality improvement studies of ESC have been compelling, the question at issue is whether the Eat, Sleep, Console care approach can reduce the time until infants with NOWS are medically ready to go home from the hospital in a wide variety of hospital settings—and, most importantly, whether it can do so safely.
To find out, the ESC-NOW team enrolled 1,305 infants with NOWS who were born after at least 36 weeks gestation. The study’s young participants were largely representative of infants with NOWS in the U.S., although non-Hispanic Black and Hispanic infants were slightly overrepresented. The babies were born at one of 26 U.S. hospitals, and each hospital was randomly assigned to transition from usual care using the Finnegan tool to the ESC care approach at a designated time.
Each hospital had a three-month transition period between the usual care and the ESC to allow clinical teams time to train on the new approach. The trial primarily aimed to understand if there was a significant difference in how long newborns with NOWS spent in the hospital before being medically ready for discharge between those receiving usual care versus those receiving ESC care. Researchers also assessed infants for safety, tracking both safety events that occurred during the hospital stay and events that occurred after the baby left the hospital, such as non-accidental trauma or death during an infant’s first three months.
The reported results reflect 837 of the 1,305 infants, who met the study definition of being medically ready for discharge. Infants who were discharged before meeting the study criteria, which were informed by the 2012 American Academy of Pediatrics recommendations for monitoring of infants with NOWS, were not included in the primary analysis.
Among the 837 infants, those who received ESC care were medically ready for discharge significantly sooner than those who received usual care. On average, they were medically ready to go home after about eight days compared to almost 15 days for the usual care group.
Many fewer infants in the ESC care group were treated with opioids compared to the usual care group (19.5 percent versus 52.0 percent). In more good news for ESC care, there was no difference in safety outcomes through the first three months despite the shorter hospital stays and reduced opioid treatment in the hospital. Infants who were cared for using the ESC care approach were no more likely to visit the doctor’s office, emergency room, or hospital after being discharged from the hospital.
More long-term study is needed to evaluate these children over months and years as they continue to develop and grow. Many of the infants in this study will be evaluated for the first two years of life to assess the long-term impact of ESC care on development and other outcomes. These findings offer encouraging early evidence that the ESC care approach is safe and effective. Although there was some variability in the outcomes, this study also shows that this approach can work well across diverse hospitals and communities.
The ESC-NOW trial is just one portion of the NIH Heal Initiative’s ACT NOW program, focused on gathering scientific evidence on how to care for babies with NOWS. Other studies are evaluating how to safely wean babies who do receive treatment with medication off opioids more quickly. The ACT NOW Longitudinal Study also will enroll at least 200 babies with prenatal opioid exposure and another 100 who were not exposed to better understand the long-term implications of early opioid exposure.
I’ve been anxious to see the results of the ESC-NOW study for a few months. It’s been worth the wait. The results show that we’re headed in the right direction with learning how best to treat NOWS and help to improve the lives of these young children and their families in the months and years ahead.
References:
[1] Eat, Sleep, Console Approach versus usual care for neonatal opioid withdrawal. Young LW, Ounpraseuth ST, Merhar SL, Newman S, Snowden JN, Devlin LA, et al. NEJM, 2023 Apr 30 [Published online ahead of print]
[2] An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Grossman MR, Berkwitt AK, Osborn RR, Xu Y, Esserman DA, Shapiro ED, Bizzarro MJ. Pediatrics. 2017 Jun;139(6):e20163360.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
“Eat, Sleep, Console” reduces hospital stay and need for medication among opioid-exposed infants, NIH news release, May 1, 2023
Helping to End Addiction Long-term® (HEAL) Initiative (NIH)
Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW)
Environmental Influences on Child Health Outcomes (ECHO) Program (NIH)
Leslie Young (The University of Vermont, Larner College of Medicine, Burlington)
NIH Support: The Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Center for Advancing Translational Sciences; Office of the Director
NIH HEAL Initiative Meets People Where They Are
Posted on by Rebecca Baker, Ph.D., NIH Helping to End Addiction Long-term® (HEAL) Initiative

The opioid crisis continues to devastate communities across America. Dangerous synthetic opioids, like fentanyl, have flooded the illicit drug supply with terrible consequences. Tragically, based on our most-recent data, about 108,000 people in the U.S. die per year from overdoses of opioids or stimulants [1]. Although this complex public health challenge started from our inability to treat pain effectively, chronic pain remains a life-altering problem for 50 million Americans.
To match the size and complexity of the crisis, in 2018 NIH developed the NIH Helping to End Addiction Long-term® (HEAL) Initiative, an aggressive effort involving nearly all of its 27 institutes and centers. Through more than 1,000 research projects, including basic science, clinical testing of new and repurposed drugs, research with communities, and health equity research, HEAL is dedicated to building a new future built on hope.
In this future:
- A predictive tool used during a health visit personalizes treatment for back pain. The tool estimates the probability that a person will benefit from physical therapy, psychotherapy, or surgery.
- Visits to community health clinics and emergency departments serve as routine opportunities to prevent and treat opioid addiction.
- Qualified school staff and pediatricians screen all children for behavioral and other mental health conditions that increase risk for harmful developmental outcomes, including opioid misuse.
- Infants born exposed to opioids during a mother’s pregnancy receive high-quality care—setting them up for a healthy future.
Five years after getting started (and interrupted by a global pandemic), HEAL research is making progress toward achieving this vision. I’ll highlight three ways in which scientific solutions are meeting people where they are today.
A Window of Opportunity for Treatment in the Justice System
Sadly, jails and prisons are “ground zero” for the nation’s opioid crisis. Eighty-five percent of people who are incarcerated have a substance use disorder or a history of substance use. Our vision at HEAL is that every person in jail, prison, or a court-supervised program receives medical care, which includes effective opioid use disorder treatment.
Some research results already are in supporting this approach: A recent HEAL study learned that individuals who had received addiction treatment while in one Massachusetts jail were about 30 percent less likely to be arrested, arraigned, or incarcerated again compared with those incarcerated during the same time period in a neighboring jail that did not offer treatment [2]. Research from the HEAL-supported Justice Community Opioid Innovation Network also is exploring public perceptions about opioid addiction. One such survey showed that most U.S. adults see opioid use disorder as a treatable medical condition rather than as a criminal matter [3]. That’s hopeful news for the future.
A Personalized Treatment Plan for Chronic Back Pain
Half of American adults live with chronic back pain, a major contributor to opioid use. The HEAL-supported Back Pain Consortium (BACPAC) is creating a whole-system model for comprehensive testing of everything that contributes to chronic low back pain, from anxiety to tissue damage. It also includes comprehensive testing of promising pain-management approaches, including psychotherapy, antidepressants, or surgery.
Refining this whole-system model, which is nearing completion, includes finding computer-friendly ways to describe the relationship between the different elements of pain and treatment. That might include developing mathematical equations that describe the physical movements and connections of the vertebrae, discs, and tendons.
Or it might include an artificial intelligence technique called machine learning, in which a computer looks for patterns in existing data, such as electronic health records or medical images. In keeping with HEAL’s all-hands-on-deck approach, BACPAC also conducts clinical trials to test new (or repurposed) treatments and develop new technologies focused on back pain, like a “wearable muscle” to help support the back.
Harnessing Innovation from the Private Sector
The HEAL research portfolio spans basic science to health services research. That allows us to put many shots on goal that will need to be commercialized to help people. Through its research support of small businesses, HEAL funding offers a make-or-break opportunity to advance a great idea to the marketplace, providing a bridge to venture capital or other larger funding sources needed for commercialization.
This bridge also allows HEAL to invest directly in the heart of innovation. Currently, HEAL funds nearly 100 such companies across 20 states. While this is a relatively small portion of all HEAL research, it is science that will make a difference in our communities, and these researchers are passionate about what they do to build a better future.
A couple of current examples of this research passion include: delivery of controlled amounts of non-opioid pain medications after surgery using a naturally absorbable film or a bone glue; immersive virtual reality to help people with opioid use disorder visualize the consequences of certain personal choices; and mobile apps that support recovery, taking medications, or sensing an overdose.
In 2023, HEAL is making headway toward its mission to accelerate development of safe, non-addictive, and effective strategies to prevent and treat pain, opioid misuse, and overdose. We have 314 clinical trials underway and 41 submissions to the Food and Drug Administration to begin clinical testing of investigational new drugs or devices: That number has doubled in the last year. More than 100 projects alone are addressing back pain, and more than 200 projects are studying medications for opioid use disorder.
The nation’s opioid crisis is profoundly difficult and multifaceted—and it won’t be solved with any single approach. Our research is laser-focused on its vision of ending addiction long-term, including improving pain management and expanding access to underused, but highly effective, addiction medications. Every day, we imagine a better future for people with physical and emotional pain and communities that are hurting. Hundreds of researchers and community members across the country are working to achieve a future where people and communities have the tools they need to thrive.
References:
[1] Provisional drug overdose death counts. Ahmad FB, Cisewski JA, Rossen LM, Sutton P. National Center for Health Statistics. 2023.
[2] Recidivism and mortality after in-jail buprenorphine treatment for opioid use disorder. Evans EA, Wilson D, Friedmann PD. Drug Alcohol Depend. 2022 Feb 1;231:109254.
[3] Social stigma toward persons with opioid use disorder: Results from a nationally representative survey of U.S. adults. Taylor BG, Lamuda PA, Flanagan E, Watts E, Pollack H, Schneider J. Subst Use Misuse. 2021;56(12):1752-1764.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
NIH Helping to End Addiction Long-term® (HEAL) Initiative
Video: The NIH HEAL Initiative–HEAL Is Hope
Justice Community Opioid Innovation Network (HEAL)
Back Pain Consortium Research Program (HEAL)
NIH HEAL Initiative 2023 Annual Report (HEAL)
Small Business Programs (HEAL)
Rebecca Baker (HEAL)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 28th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Updating an Rx for Progress
Posted on by Lawrence Tabak, D.D.S., Ph.D.

On April 11, I took part in an afternoon plenary session titled: “State of the Science: Updates from the National Institutes of Health.” Joining me on the stage were my NIH colleagues Nora Volkow, director, National Institute on Drug Abuse; and George Koob, director, National Institute on Alcohol Abuse and Alcoholism. I was able to update everyone on the research progress being made by the NIH Helping to End Addiction (HEAL) Initiative. The initiative, through its support of more than 1,000 projects across the nation, aims to prevent addiction through enhanced pain management, while seeking better ways to improve prevention and treatment for opioid misuse disorder and addiction. The Rx and Illicit Drug Summit 2023 took place on April 11-13 at the Georgia World Congress Center. Credit: Pierce Harman, HMP Global, Malvern, PA.
Connecting the Dots: Oral Infection to Rheumatoid Arthritis
Posted on by Lawrence Tabak, D.D.S., Ph.D.
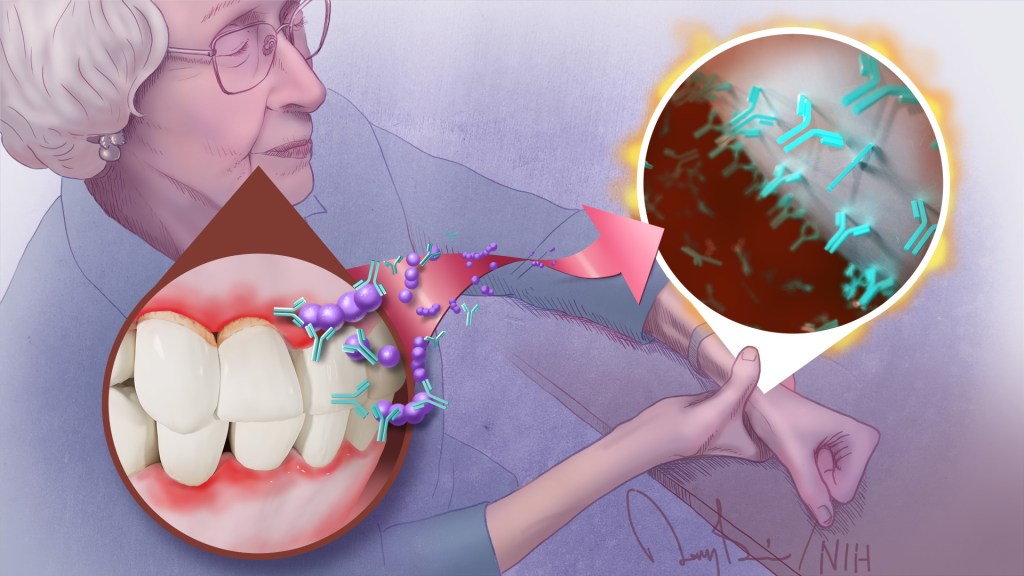
To keep your teeth and gums healthy for a lifetime, it’s important to brush and floss each day and see your dentist regularly. But what you might not often stop to consider is how essential good oral health really is to your overall well-being. The mouth, after all, is connected to the rest of the body, and oral infections can contribute to problems elsewhere.
A good case in point comes from a study just published in the journal Science Translational Medicine. The study, though small, offers some of the most convincing evidence yet for a direct link between gum, or periodontal, disease and the rheumatoid arthritis that flares most commonly in the hands, wrists, and knees [1]. If confirmed in larger follow-up studies, the finding suggests that one way for people with both diseases to contend with painful arthritic flare-ups will be to prevent them by practicing good oral hygiene and controlling their periodontal disease.
For many years, there had been suggestions that the oral bacteria causing periodontal disease might contribute to rheumatoid arthritis. For instance, past studies have found that periodontal disease occurs even more often in people with rheumatoid arthritis. People with both conditions also tend to have more severe arthritic symptoms that can be more stubbornly resistant to treatment.
What’s been missing is the precise underlying mechanisms to confirm the connection. To help connect the dots, a research team, which included Dana Orange, Rockefeller University, New York, NY, and William Robinson, Stanford University, Stanford, CA, decided to look closer.
They looked first in the blood, not directly at an arthritic joint or an inflamed periodontium, the tissues that hold a tooth in place. They were interested in whether telltale changes in the blood of people with rheumatoid arthritis correlated with the start of another painful flare-up in one or more of their joints.
One of those possible changes involves proteins that carry a particular chemical modification that places the amino acid citrulline on their surface. These citrulline-marked proteins are found in many parts of the human body, including the joints. Intriguingly, they also are present on bacteria, including those in the mouth.
Because of this bacterial connection, the researchers looked in the blood for a specific set of antibodies known as ACPAs, short for anti-citrullinated protein antibodies. They recognize citrullinated proteins that are foreign to the body and mark them for attack.
But the attack isn’t always perfectly aimed, and studies have shown the presence of ACPAs in the joints of people with rheumatoid arthritis is associated with increasing disease activity and more frequent arthritis flares. Periodontal disease, too, is especially common in people with rheumatoid arthritis who have abnormally high levels of circulating ACPAs.
In the new study, the researchers followed five women with rheumatoid arthritis for one to four years. Two of them had severe periodontal disease while the other three had no periodontal disease.
Each week, the study volunteers provided a small blood sample for researchers to study changes at the level of RNA, the genetic material that encodes proteins. They also studied changes in certain immune cells, along with any changes in their medication, dental care, or arthritis symptoms. For additional information, they also looked at blood and joint fluid samples from 67 other people with and without arthritis, including individuals with healthy gums or mild, moderate, or severe periodontal disease.
Overall, the evidence shows that people with more severe periodontal disease experienced repeated influxes of oral bacteria into their blood even when they hadn’t had a recent dental procedure. These findings suggested that when their inflamed gums became more damaged and “leaky,” bacteria in the mouth could spill into the bloodstream.
The researchers also found that those oral invaders carried many citrullinated proteins. Once they got into the bloodstream, inflammatory immune cells detected them and released ACPAs.
The researchers showed in the lab that those antibodies bind the same oral bacteria detected in the blood of people with periodontal disease and rheumatoid arthritis. In fact, those with both conditions had a wide variety of genetically distinct ACPAs, as would be expected if their immune systems were challenged repeatedly over time with oral bacteria.
The overarching idea is that these antibodies prime the immune system to attack oral bacteria. But after it gets started, the attack mistakenly expands and targets citrullinated proteins in the joints. That triggers a flare-up in a joint and the characteristic inflammation, stiffness, and joint damage.
While more study is needed to fill in the molecular details, this discovery raises an encouraging possibility. Taking care of your teeth and periodontal disease isn’t just a wise idea to maintain good oral health over a lifetime. For some of the approximately 1 million Americans with rheumatoid arthritis, it may help to manage and perhaps even prevent a painful flare-up in one or more of their affected joints.
Reference:
[1] Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis. Brewer RC, Lanz TV, Hale CR, Sepich-Poore GD, Martino C, Swafford AD, Carroll TS, Kongpachith S, Blum LK, Elliott SE, Blachere NE, Parveen S, Fak J, Yao V, Troyanskaya O, Frank MO, Bloom MS, Jahanbani S, Gomez AM, Iyer R, Ramadoss NS, Sharpe O, Chandrasekaran S, Kelmenson LB, Wang Q, Wong H, Torres HL, Wiesen M, Graves DT, Deane KD, Holers VM, Knight R, Darnell RB, Robinson WH, Orange DE. Sci Transl Med. 2023 Feb 22;15(684):eabq8476.
Links:
Rheumatoid Arthritis (National Institute of Arthritis and Musculoskeletal and Skin Diseases)
Periodontal (Gum) Disease (National Institute of Dental and Craniofacial Research/NIH)
Oral Hygiene (NIDCR)
Dana Orange (Rockefeller University, New York NY)
Robinson Lab (Stanford University, Stanford, CA)
NIH Support: National Institute of Arthritis and Musculoskeletal and Skin Diseases; National Institute of Allergy and Infectious Diseases; National Human Genome Research Institute; National Institute of General Medical Sciences; National Center for Advancing Translational Sciences; National Cancer Institute
Tackling Complex Scientific Questions Requires a Team Approach
Posted on by Nora D. Volkow, M.D., National Institute on Drug Abuse

During the COVID-19 pandemic, we have seen unprecedented, rapid scientific collaboration, as experts around the world in discrete, previously disconnected fields, have found ways to collaborate to face a common cause. For example, physicists helped respiratory specialists understand how virus particles could spread in air, leading to improved mitigation strategies. Specialists in cardiovascular science, neuroscience, immunology, and other fields are now working together to understand and address Long COVID. Over the past two years, we have also seen remarkable international sharing of epidemiological data and information on effects of vaccines.
Science is increasingly a team activity, which is true for many fields, not just biomedicine. The professional diversity of research teams reflects the increased complexity of the questions science is called upon to answer. This is especially obvious in the study of the brain, which is the most complex system known to us.
The NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, with the goal of vastly enhancing neuroscience through new technologies, includes research teams with neuroscientists, engineers, mathematicians, physicists, data scientists, ethicists, and more. Nearly half (47 percent) of grant awards have multiple principal investigators.
Besides the BRAIN Initiative, other multi-institute NIH research projects are applying team science to complex research questions, such as those related to neurodevelopment, addiction, and pain. The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, created a team-based research framework to advance promising pain therapeutics quickly to clinical testing.
In the Adolescent Brain Cognitive Development (ABCD) study, which is led by NIDA in close partnership with NIH’s National Institute on Alcohol Abuse and Alcoholism (NIAAA), and other NIH institutes, 21 research centers are collecting behavioral, biospecimen, and neuroimaging data from 11,878 children from age 10 through their teens. Teams led by experts in adolescent psychiatry, developmental psychology, and pediatrics interview participants and their families. These experts then gather a battery of health metrics from psychological, cognitive, sociocultural, and physical assessments, including collection and analysis of various kinds of biospecimens (blood, saliva). Further, experts in biophysics gather information on the structure and function of participants’ brains every two years.
A similar study of young children in the first decade of life beginning with the prenatal period, the HEALthy Brain and Child Development (HBCD) study, supported by HEAL, NIDA, and several other NIH institutes and centers, is now underway at 25 research sites across the country. A range of scientific specialists, similar to that in the ABCD study, is involved in this effort. In this case, they are aided by experts in obstetric care and in infant neuroimaging.
For both of these studies, teams of data scientists validate and curate all the information generated and make it available to researchers across the world. This makes it possible to investigate complex questions such as human neurodevelopmental diversity and the effects of genes and social experiences and their relation to mental health. More than half of the publications using ABCD data have been authored by non-ABCD investigators taking advantage of the open-access format.
Yet, institutions that conduct and fund science—including NIH—have been slow to support and reward collaboration. Because authorship and funding are so important in tenure and promotion decisions at universities, for example, an individual’s contribution to larger, multi-investigator projects on which they may not be the grantee or lead author on a study publication may carry less weight.
For this reason, early-career scientists may be particularly reluctant to collaborate on team projects. Among the recommendations of a 2015 National Academies of Sciences, Engineering, and Medicine (NASEM) report, Enhancing the Effectiveness of Team Science, was that universities and other institutions should find effective ways to give credit for team-based work to assist promotion and tenure committees.
The strongest teams will be diverse in other respects, not just scientific expertise. Besides more actively fostering productive collaborations across disciplines, NIH is making a more concerted effort to promote racial equity and inclusivity in our research workforce, both through the NIH UNITE Initiative and through Institute-specific initiatives like NIDA’s Racial Equity Initiative.
To promote diversity, inclusivity, and accessibility in research, the BRAIN Initiative recently added a requirement in most of its funding opportunity announcements (FOAs) that has applicants include a Plan for Enhancing Diverse Perspectives (PEDP) in the proposed research. The PEDPs are evaluated and scored during the peer review as part of the holistic considerations used to inform funding decisions. These long-overdue measures will not only ensure that NIH-funded science is more diverse, but they are also important steps toward studying and addressing social determinants of health and the health disparities that exist for so many conditions.
Increasingly, scientific discovery is as much about exploring new connections between different kinds of researchers as it is about finding new relationships among different kinds of scientific databases. The challenges before us are great—ending the COVID pandemic, finding a solution to the addiction and overdose crisis, and so many others—and increased collaboration between scientists will give us the greatest chance to successfully overcome these challenges.
Links:
Nora Volkow’s Blog (National Institute on Drug Abuse/NIH)
Adolescent Brain Cognitive Development Study
Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Racial Equity Initiative (NIDA)
Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 13th in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
Previous Page Next Page