type 2
Uncovering Disease-Driving Events that Lead to Type 2 Diabetes
Posted on by Dr. Monica M. Bertagnolli
Nearly 35 million people in communities across the U.S. have type 2 diabetes (T2D), putting them at increased risk for a wide range of serious health complications, including vision loss, kidney failure, heart disease, stroke, and premature death.1 While we know a lot about the lifestyle and genetic factors that influence diabetes risk and steps that can help prevent or control it, there’s still a lot to learn about the precise early events in the body that drive this disease.
When you have T2D, the insulin-producing beta cells in your pancreas don’t release insulin in the way that they should. As a result, blood sugar doesn’t enter your cells, and its levels in the bloodstream go up. What’s less clear is exactly what happens to cause beta cells and the cell clusters where they’re found (called islets) to malfunction in the first place. However, I’m encouraged by some new NIH-supported research in Nature that used various large datasets to identify key signatures of islet dysfunction in people with T2D.2
Earlier studies have linked about 400 sites in the human genome to an increased risk for T2D. But most of them—more than 9 in 10—are primarily in noncoding stretches of DNA that control genes. As a result, it’s been hard to figure out exactly how those genetic variants that increase risk in the general population lead to the changes in individuals who go on to develop T2D.
In the new study, a team led by Marcela Brissova and Alvin C. Powers, Vanderbilt University Medical Center, Nashville, and Stephen C.J. Parker, University of Michigan, Ann Arbor, used sophisticated analytic approaches to study changes within pancreatic tissues and islets taken from donors who’d had early-stage T2D at the time of their death. They included tissues from donors without T2D to serve as a comparison.
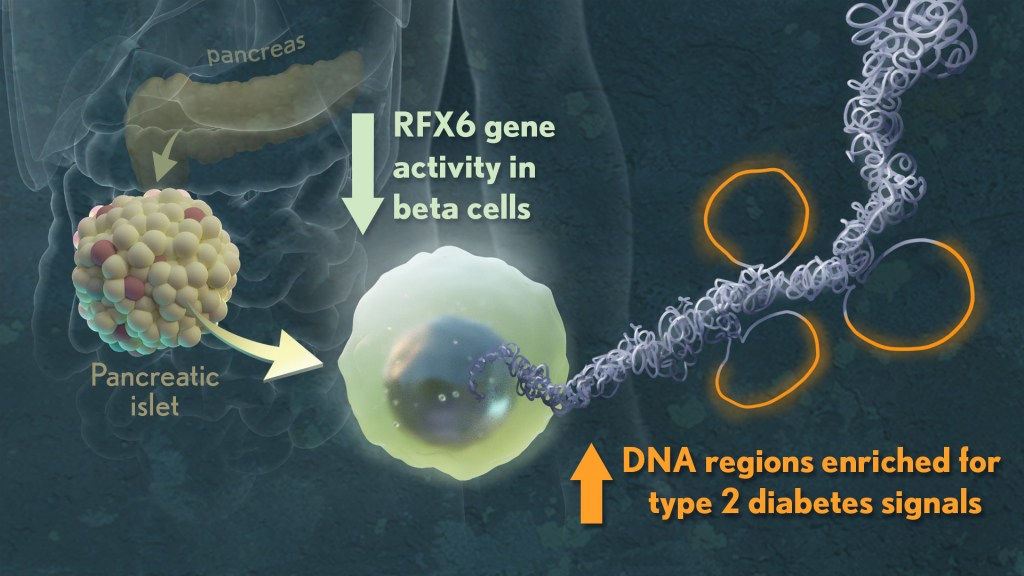
To get a better understanding, they looked at the tissues in multiple ways, studying differences in their basic physiology, gene activity, and cellular-level structures. By integrating data on these observed differences with other types of data from prior studies, they showed that impaired function of beta cells is a hallmark of early T2D, reinforcing prior evidence. Other pancreatic islet cell types appeared mostly unchanged.
Their studies also showed that alterations in a particular gene network are key in early-stage T2D. The network, controlled by a protein called RFX6, cause pancreatic beta cells to malfunction. The researchers performed additional studies that showed lowering RFX6 levels led beta cells to secrete less insulin. Lower RFX6 levels also led to structural changes in the DNA, specifically in sites that have known links to diabetes risk. They expanded this finding by doing a population-scale genetic analysis. Using genetic information for more than 500,000 volunteers available in the UK biobank, they showed a causal link between lower levels of RFX6 and T2D.
Further study is needed to understand what’s behind the initial changes in RFX6. The researchers also want to explore further whether RFX6 might be a promising target for new treatments to prevent or reverse early-stage molecular and functional defects in the beta cell that underlie T2D. The researchers note that they have made all the data publicly available through user-friendly and interactive web portals, in hopes it will lead to more answers for the millions already affected by T2D and so many others who may be at risk.
References:
[2] JT Walker, DC Saunders, V Rai, et al. Genetic risk converges on regulatory networks mediating early type 2 diabetes. Nature. DOI: 10.1038/s41586-023-06693-2 (2023).
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases, National Heart, Lung, and Blood Institute, National Eye Institute, National Institute of General Medical Sciences
The Diabetes Threat
Posted on by Dr. Francis Collins
The number of Americans diagnosed with type 2 diabetes rose from 1.5 million in 1958 to 18.8 million in 2010. That’s an increase of epidemic proportions. Even more disturbing, another 7 million Americans have type 2 diabetes, but don’t know it and, consequently, can’t take steps to control the disease. Altogether, over 8% of the U.S. population now has this potentially deadly metabolic condition.
- Type 2 diabetes wreaks havoc on the body by raising the levels of glucose in the blood, increasing the risk of blindness, heart disease, kidney failure, nerve damage, and even Alzheimer’s disease.
- Pre-diabetes is a condition in which blood glucose levels are higher than normal, but not high enough to be called diabetes. 79 million U.S. adults age 20 and older have pre-diabetes.
- NIH studies have shown that losing just 6–7% of body weight and increasing physical activity can prevent or delay pre-diabetes from progressing to diabetes. 85% of people with diabetes are overweight.

