schizophrenia
Teaching Computers to “See” the Invisible in Living Cells
Posted on by Dr. Francis Collins
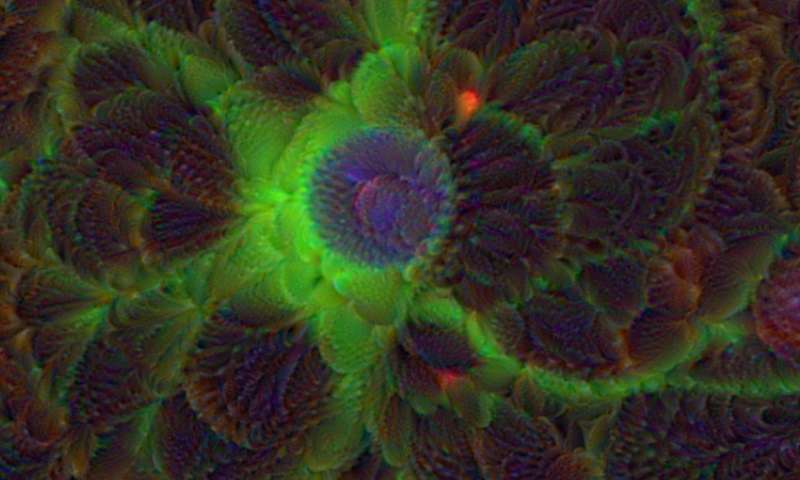
Credit: Steven Finkbeiner, University of California, San Francisco and the Gladstone Institutes
For centuries, scientists have trained themselves to look through microscopes and carefully study their structural and molecular features. But those long hours bent over a microscope poring over microscopic images could be less necessary in the years ahead. The job of analyzing cellular features could one day belong to specially trained computers.
In a new study published in the journal Cell, researchers trained computers by feeding them paired sets of fluorescently labeled and unlabeled images of brain tissue millions of times in a row [1]. This allowed the computers to discern patterns in the images, form rules, and apply them to viewing future images. Using this so-called deep learning approach, the researchers demonstrated that the computers not only learned to recognize individual cells, they also developed an almost superhuman ability to identify the cell type and whether a cell was alive or dead. Even more remarkable, the trained computers made all those calls without any need for harsh chemical labels, including fluorescent dyes or stains, which researchers normally require to study cells. In other words, the computers learned to “see” the invisible!
Creative Minds: Mapping the Biocircuitry of Schizophrenia and Bipolar Disorder
Posted on by Dr. Francis Collins
As a graduate student in the 1980s, Bruce Yankner wondered what if cancer-causing genes switched on in non-dividing neurons of the brain. Rather than form a tumor, would those genes cause neurons to degenerate? To explore such what-ifs, Yankner spent his days tinkering with neural cells, using viruses to insert various mutant genes and study their effects. In a stroke of luck, one of Yankner’s insertions encoded a precursor to a protein called amyloid. Those experiments and later ones from Yankner’s own lab showed definitively that high concentrations of amyloid, as found in the brains of people with Alzheimer’s disease, are toxic to neural cells [1].
The discovery set Yankner on a career path to study normal changes in the aging human brain and their connection to neurodegenerative diseases. At Harvard Medical School, Boston, Yankner and his colleague George Church are now recipients of an NIH Director’s 2016 Transformative Research Award to apply what they’ve learned about the aging brain to study changes in the brains of younger people with schizophrenia and bipolar disorder, two poorly understood psychiatric disorders.
Creative Minds: Modeling Neurobiological Disorders in Stem Cells
Posted on by Dr. Francis Collins
Most neurological and psychiatric disorders are profoundly complex, involving a variety of environmental and genetic factors. Researchers around the world have worked with patients and their families to identify hundreds of possible genetic leads to learn what goes wrong in autism spectrum disorder, schizophrenia, and other conditions. The great challenge now is to begin examining this growing cache of information more systematically to understand the mechanism by which these gene variants contribute to disease risk—potentially providing important information that will someday lead to methods for diagnosis and treatment.
Meeting this profoundly difficult challenge will require a special set of laboratory tools. That’s where Feng Zhang comes into the picture. Zhang, a bioengineer at the Broad Institute of MIT and Harvard, Cambridge, MA, has made significant contributions to a number of groundbreaking research technologies over the past decade, including optogenetics (using light to control brain cells), and CRISPR/Cas9, which researchers now routinely use to edit genomes in the lab [1,2].
Zhang has received a 2015 NIH Director’s Transformative Research Award to develop new tools to study multiple gene variants that might be involved in a neurological or psychiatric disorder. Zhang draws his inspiration from nature, and the microscopic molecules that various organisms have developed through the millennia to survive. CRISPR/Cas9, for instance, is a naturally occurring bacterial defense system that Zhang and others have adapted into a gene-editing tool.
Big Data and Imaging Analysis Yields High-Res Brain Map
Posted on by Dr. Francis Collins

Caption: Map of 180 areas in the left and right hemispheres of the cerebral cortex.
Credit: Matthew F. Glasser, David C. Van Essen, Washington University Medical School, Saint Louis, Missouri
Neuroscientists have been working for a long time to figure out how the human brain works, and that has led many through the years to attempt to map its various regions and create a detailed atlas of their complex geography and functions. While great progress has been made in recent years, existing brain maps have remained relatively blurry and incomplete, reflecting only limited aspects of brain structure or function and typically in just a few people.
In a study reported recently in the journal Nature, an NIH-funded team of researchers has begun to bring this map of the human brain into much sharper focus [1]. By combining multiple types of cutting-edge brain imaging data from more than 200 healthy young men and women, the researchers were able to subdivide the cerebral cortex, the brain’s outer layer, into 180 specific areas in each hemisphere. Remarkably, almost 100 of those areas had never before been described. This new high-resolution brain map will advance fundamental understanding of the human brain and will help to bring greater precision to the diagnosis and treatment of many brain disorders.
Study May RAISE Standard for Treating First Psychotic Episode
Posted on by Dr. Francis Collins
Each year, about 100,000 American adolescents and young adults, their lives and dreams ahead of them, experience their first episode of psychosis, a symptom of schizophrenia and other mental illnesses characterized by dramatic changes in perception, personality, and ability to function [1]. This often-terrifying experience, which can last for months, will prompt some to seek help from mental health professionals, whose services can in many situations help them get back on track and reduce the risk of relapse. Still, for far too many young people and their families, the search for help is riddled with long delays, contradictory information, and inadequate treatment in a mental health system whose resources have been stretched thin.
There’s got to be a better way to reach more of these young people, and, now, results of a major NIH-supported clinical study point to a possible way to get there [2]. In this large study, published in the American Journal of Psychiatry, teams of mental health specialists partnered with young people and their families to create individualized treatment plans. After two years of follow-up, researchers found that this personalized, team-based approach to care had helped more young people stick with treatment, feel better about their quality of life, return to school and work, and seek follow-up help than standard care involving a single clinician.
Many studies show the longer that people with psychotic episodes go untreated, the harder it is to stabilize their symptoms and the more problems they develop. A common presentation is schizophrenia, a persistent, severe brain disorder that often can be diagnosed only months or even years after a first psychotic episode. Schizophrenia affects 1.1 percent of Americans ages 18 and older, and currently accounts for about 30 percent of all spending on mental health treatment [3].
Creative Minds: Making Sense of Stress and the Brain
Posted on by Dr. Francis Collins
Right behind your forehead lies the most recently evolved region of the human brain: the prefrontal cortex (PFC). It’s a major control center for abstract thinking, thought analysis, working memory, planning, decision making, regulating emotions, and many of the things we most strongly associate with being human. But in times of stress, the PFC is literally taken offline, allowing more primitive parts of the brain to take over.
Amy Arnsten, a neuroscientist at the Yale School of Medicine, New Haven, CT, has pioneered the study of stress on the brain [1] and how impaired regulation of stress response in the PFC contributes to neurological disorders, such as Attention Deficit Hyperactivity Disorder (ADHD), schizophrenia [2, 3], and Alzheimer’s disease [4]. In these disorders, cells in the PFC are negatively affected, while those in the primary sensory cortex, a more primitive part of the brain that processes vision and sound, are thought to remain relatively unscathed. With support from a 2013 NIH Director’s Pioneer Award, Arnsten hopes to uncover why the PFC is more vulnerable to disease than the primary sensory cortex—and how we might be able to prevent or reverse damage to these circuits.
Exploring the Complex Genetics of Schizophrenia
Posted on by Dr. Francis Collins
Schizophrenia is one of the most prevalent, tragic, and frustrating of all human illnesses, affecting about 1% of the human population, or 2.4 million Americans [1]. Decades of research have failed to provide a clear cause in most cases, but family clustering has suggested that inheritance must play some role. Over the last five years, multiple research projects known as genome-wide association studies (GWAS) have identified dozens of common variations in the human genome associated with increased risk of schizophrenia [2]. However, the individual effects of these variants are weak, and it’s often not been clear which genes were actually affected by the variations. Now, advances in DNA sequencing technology have made it possible to move beyond these association studies to study the actual DNA sequence of the protein-coding region of the entire genome for thousands of individuals with schizophrenia. Reports just published have revealed a complex constellation of rare mutations that point to specific genes—at least in certain cases.
Previous Page






