bacteria
Portable System Uses Light to Diagnose Bacterial Infections Faster
Posted on by Dr. Francis Collins

Caption: PAD system. Left, four optical testing cubes (blue and white) stacked on the electronic base station (white with initials); right, a smartphone with a special app to receive test results transmitted by the electronic base station.
Credit: Park et al. Sci. Adv. 2016
Every year, hundreds of thousands of Americans acquire potentially life-threatening bacterial infections while in the hospital, nursing home, or other health-care settings [1]. Such infections can be caused by a variety of bacteria, which may respond quite differently to different antibiotics. To match a patient with the most appropriate antibiotic therapy, it’s crucial to determine as quickly as possible what type of bacteria is causing his or her infection. In an effort to improve that process, an NIH-funded team is working to develop a point-of-care system and smartphone app aimed at diagnosing bacterial infections in a faster, more cost-effective manner.
The portable new system, described recently in the journal Science Advances, uses a novel light-based method for detecting telltale genetic sequences from bacteria in bodily fluids, such as blood, urine, or drainage from a skin abscess. Testing takes place within small, optical cubes that, when placed on an electronic base station, deliver test results within a couple of hours via a simple readout sent directly to a smartphone [2]. When the system was tested on clinical samples from a small number of hospitalized patients, researchers found that not only did it diagnose bacterial infections about as accurately and more swiftly than current methods, but it was also cheaper. This new system can potentially also be used to test for the presence of antibiotic-resistant bacteria and contamination of medical devices.
Cool Videos: Another Kind of Art Colony
Posted on by Dr. Francis Collins
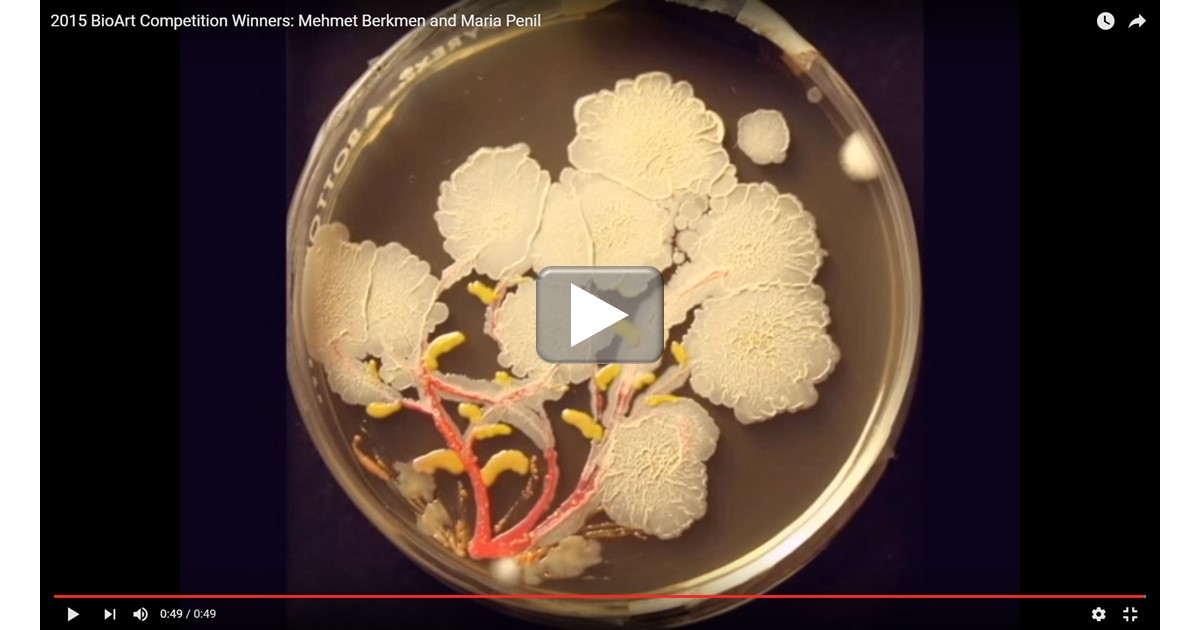 As long as researchers have been growing bacteria on Petri dishes using a jelly-like growth medium called agar, they have been struck by the interesting colors and growth patterns that microbes can produce from one experiment to the next. In the 1920s, Sir Alexander Fleming, the Scottish biologist who discovered penicillin, was so taken by this phenomenon that he developed his own palette of bacterial “paints” that he used in his spare time to create colorful pictures of houses, ballerinas, and other figures on the agar [1].
As long as researchers have been growing bacteria on Petri dishes using a jelly-like growth medium called agar, they have been struck by the interesting colors and growth patterns that microbes can produce from one experiment to the next. In the 1920s, Sir Alexander Fleming, the Scottish biologist who discovered penicillin, was so taken by this phenomenon that he developed his own palette of bacterial “paints” that he used in his spare time to create colorful pictures of houses, ballerinas, and other figures on the agar [1].
Fleming’s enthusiasm for agar art lives on among the current generation of microbiologists. In this short video, the agar (yellow) is seeded with bacterial colonies and, through the magic of time-lapse photography, you can see the growth of the colonies into what appears to be a lovely bouquet of delicate flowers. This piece of living art, developing naturally by bacterial colony expansion over the course of a week or two, features members of three bacterial genera: Serratia (red), Bacillus (white), and Nesterenkonia (light yellow).
LabTV: Curious about Computer Modeling of Proteins
Posted on by Dr. Francis Collins
In many ways, Josh Carter is a typical college student, with a hectic schedule packed with classes and social activities. But when he enters a structural biology lab at Montana State University in Bozeman, Carter encounters an even faster paced world in which molecular interactions can be measured in femtoseconds—that is, 1 millionth of 1 billionth of 1 second.
Working under the expert eye of principal investigator Blake Wiedenheft, Carter is applying his computational skills to X-ray crystallography data to model the structures of various proteins, as well as to chart their evolution over time and map their highly dynamic interactions with other proteins and molecules. This basic science work is part of this NIH-funded lab’s larger mission to understand how bacteria defend themselves from the viruses that try to infect them. It’s a fascinating area of science with a wide range of potential applications, from treating diseases that arise from imbalances in the microbiome (the communities of microbes that live in and on our bodies) to developing new methods for gene editing and programmable control of gene expression.
Snapshots of Life: A Kaleidoscope of Worms
Posted on by Dr. Francis Collins
What might appear to be a view inside an unusual kaleidoscope is actually a laboratory plate full of ravenous roundworms (Caenorhabditis elegans) as seen through a microscope. Tens of thousands of worms (black), each about 1 millimeter in length at adulthood, are grazing on a field of bacteria beneath them. The yellow is a jelly-like growth medium called agar that feeds the bacteria, and the orange along the borders was added to enhance the sunburst effect.
The photo was snapped and stylized by NIH training grantee Adam Brown, a fourth-year Ph.D. student in the lab of David Biron at the University of Chicago. Brown uses C. elegans to study the neurotransmitter serotonin, a popular drug target in people receiving treatment for depression and other psychiatric disorders. This tiny, soil-dwelling worm is a go-to model organism for neuroscientists because of its relative simplicity, short life spans, genetic malleability, and complete cell-fate map. By manipulating the different components of the serotonin-signaling system in C. elegans, Brown and his colleagues hope to better understand the most basic circuitry in the central nervous system that underlies decision making, in this case choosing to feed or forage.
Creative Minds: Bacteria, Gene Swaps, and Human Cancer
Posted on by Dr. Francis Collins

Julie Dunning Hotopp
When Julie Dunning Hotopp was a post-doctoral fellow in the early 2000s, bacteria were known for swapping bits of their DNA with other bacteria, a strategy known as lateral gene transfer. But the offloading of genes from bacteria into multicellular organisms was thought to be rare, with limited evidence that a bacterial genus called Wolbachia, which invades the cells of other organisms and takes up permanent residence, had passed off some of its DNA onto a species of beetle and a parasitic worm. Dunning Hotopp wondered whether lateral gene transfer might be a more common phenomenon than the evidence showed.
She and her colleagues soon discovered that Wolbachia had engaged in widespread lateral gene transfer with eight species of insects and nematode worms, possibly passing on genes and traits to their invertebrate hosts [1]. This important discovery put Dunning Hotopp on a research trail that now has taken a sharp turn toward human cancer and earned her a 2015 NIH Director’s Transformative Research Award. This NIH award supports exceptionally innovative research projects that are inherently risky and untested but have the potential to change fundamental research paradigms in areas such as cancer and throughout the biomedical sciences.
Gene Expression Test Aims to Reduce Antibiotic Overuse
Posted on by Dr. Francis Collins

Caption: Duke physician-scientist Ephraim Tsalik assesses a patient for a respiratory infection.
Credit: Shawn Rocco/Duke Health
Without doubt, antibiotic drugs have saved hundreds of millions of lives from bacterial infections that would have otherwise been fatal. But their inappropriate use has led to the rise of antibiotic-resistant superbugs, which now infect at least 2 million Americans every year and are responsible for thousands of deaths [1]. I’ve just come from the World Economic Forum in Davos, Switzerland, where concerns about antibiotic resistance and overuse was a topic of conversation. In fact, some of the world’s biggest pharmaceutical companies issued a joint declaration at the forum, calling on governments and industry to work together to combat this growing public health threat [2].
Many people who go to the doctor suffering from respiratory symptoms expect to be given a prescription for antibiotics. Not only do such antibiotics often fail to help, they serve to fuel the development of antibiotic-resistant superbugs [3]. That’s because antibiotics are only useful in treating respiratory illnesses caused by bacteria, and have no impact on those caused by viruses (which are frequent in the wintertime). So, I’m pleased to report that a research team, partially supported by NIH, recently made progress toward a simple blood test that analyzes patterns of gene expression to determine if a patient’s respiratory symptoms likely stem from a bacterial infection, viral infection, or no infection at all.
In contrast to standard tests that look for signs of a specific infectious agent—respiratory syncytial virus (RSV) or the influenza virus, for instance—the new strategy casts a wide net that takes into account changes in the patterns of gene expression in the bloodstream, which differ depending on whether a person is fighting off a bacterial or a viral infection. As reported in Science Translational Medicine [4], Geoffrey Ginsburg, Christopher Woods, and Ephraim Tsalik of Duke University’s Center for Applied Genomics and Precision Medicine, Durham, NC, and their colleagues collected blood samples from 273 people who came to the emergency room (ER) with signs of acute respiratory illness. Standard diagnostic tests showed that 70 patients arrived in the ER with bacterial infections and 115 were battling viruses. Another 88 patients had no signs of infection, with symptoms traced instead to other health conditions.
Creative Minds: Searching for Solutions to Chronic Infection
Posted on by Dr. Francis Collins
If you or a loved one has ever struggled with a bacterial infection that seemed to have gone away with antibiotic treatment, but then came back again, you’ll probably be interested to learn about the work of Kyle Allison. What sometimes happens when a person has an infection—for instance, a staph infection of the skin—is that antibiotics kill off the vast majority of bacteria, but a small fraction remain alive. After antibiotic treatment ends, those lurking bacterial “persisters” begin to multiply, and the person develops a chronic infection that may be very difficult and costly to eliminate.
Unlike antibiotic-resistant superbugs, bacterial persisters don’t possess any specific genetic mutations that protect them against the killing power of one particular medication or another. Rather, the survival of these bacteria depends upon their ability to enter a dormant state that allows them to hang on in the face of antibiotic treatment. It isn’t clear exactly how the bugs do it, and that’s where Kyle’s work comes in.
LabTV: Curious About the Microbiome
Posted on by Dr. Francis Collins
 When people think about the human microbiome—the scientific term for all of the microbes that live in and on our bodies—the focus is often on bacteria. But Keisha Findley, the young researcher featured in today’s LabTV video, is fascinated by a different part of the microbiome: fungi.
When people think about the human microbiome—the scientific term for all of the microbes that live in and on our bodies—the focus is often on bacteria. But Keisha Findley, the young researcher featured in today’s LabTV video, is fascinated by a different part of the microbiome: fungi.
While earning her Ph.D. at Duke University, Durham, N.C., Findley zeroed in on Cryptococcus neoformans, a common, single-celled fungus that can lead to life-threatening infections, especially in people with weakened immune systems. Now, as a postdoctoral fellow at NIH’s National Human Genome Research Institute, Bethesda, MD, she is part of an effort to survey all of the fungi, as well as bacteria, that live on healthy human skin. The goal is to get a baseline understanding of these microbial communities and then examine how they differ between healthy people and those with skin conditions such as acne, athlete’s foot, skin ulcers, psoriasis, or eczema.
LabTV: Curious About Bacteria
Posted on by Dr. Francis Collins
 Other than wondering what might be lurking in those leftovers stashed in the back of the fridge, you probably don’t think much about bacteria. But Robert Morton III—a Ph.D. candidate at Indiana University, Bloomington, and the focus of our latest LabTV profile—sure does. He’s fascinated by the complicated and even beautiful ways in which bacteria interact with their environments. In fact, scientists can learn a whole lot about biology by studying bacteria and other single-celled organisms.
Other than wondering what might be lurking in those leftovers stashed in the back of the fridge, you probably don’t think much about bacteria. But Robert Morton III—a Ph.D. candidate at Indiana University, Bloomington, and the focus of our latest LabTV profile—sure does. He’s fascinated by the complicated and even beautiful ways in which bacteria interact with their environments. In fact, scientists can learn a whole lot about biology by studying bacteria and other single-celled organisms.
Working in the NIH-funded lab of Yves Brun, Morton has spent many of his days peering through microscopes into the otherwise invisible world of bacteria. His sights are set on the relatively simple, two-component interactions that enable bacteria to sense and respond to various external factors. Each of these interactions features a histidine kinase sensor partnered with a response regulator. Specifically, Morton has focused much of his research on one particular protein thought to play a role in these interactions—a protein that he calls an “orphan” because no scientist has yet identified its partner or determined quite what it does.
Manipulating Microbes: New Toolbox for Better Health?
Posted on by Dr. Francis Collins

Caption: Bacteroides thetaiotaomicron (white) living on mammalian cells in the gut (large pink cells coated in microvilli) and being activated by exogenously added compounds (small green dots) to express specific genes, such as those encoding light-generating luciferase proteins (glowing bacteria).
Credit: Janet Iwasa, Broad Visualization Group, MIT Media Lab
When you think about the cells that make up your body, you probably think about the cells in your skin, blood, heart, and other tissues and organs. But the one-celled microbes that live in and on the human body actually outnumber your own cells by a factor of about 10 to 1. Such microbes are especially abundant in the human gut, where some of them play essential roles in digestion, metabolism, immunity, and maybe even your mood and mental health. You are not just an organism. You are a superorganism!
Now imagine for a moment if the microbes that live inside our guts could be engineered to keep tabs on our health, sounding the alarm if something goes wrong and perhaps even acting to fix the problem. Though that may sound like science fiction, an NIH-funded team from the Massachusetts Institute of Technology (MIT) in Cambridge, MA, is already working to realize this goal. Most recently, they’ve developed a toolbox of genetic parts that make it possible to program precisely one of the most common bacteria found in the human gut—an achievement that provides a foundation for engineering our collection of microbes, or microbiome, in ways that may treat or prevent disease.
Previous Page Next Page