24 Search Results for "Addiction Science"
Using Science To Solve Oral Health Inequities
Posted on by Rena D'Souza, D.D.S., M.S., Ph.D., National Institute of Dental and Craniofacial Research

At NIH, we have a front row seat to remarkable advances in science and technology that help Americans live longer, healthier lives. By studying the role that the mouth and saliva can play in the transmission and prevention of disease, the National Institute of Dental and Craniofacial Research (NIDCR) contributed to our understanding of infectious agents like the coronavirus SARS-CoV-2, the cause of COVID-19. While these and other NIH-supported advances undoubtedly can improve our nation’s health as a whole, not everyone enjoys the benefits equally—or at all. As a result, people’s health, including their oral health, suffers.
That’s a major takeaway from Oral Health in America: Advances and Challenges, a report that NIDCR recently released on the status of the nation’s oral health over the last 20 years. The report shows that oral health has improved in some ways, but people from marginalized groups —such as those experiencing poverty, people from racial and ethnic minority groups, the frail elderly, and immigrants—shoulder an unequal burden of oral disease.
At NIDCR, we are taking the lessons learned from the Oral Health in America report and using them to inform our research. It will help us to discover ways to eliminate these oral health differences, or disparities, so that everyone can enjoy the benefits of good oral health.
Why does oral health matter? It is essential for our overall health, well-being, and productivity. Untreated oral diseases, such as tooth decay and gum disease, can cause infections, pain, and tooth loss, which affect the ability to chew, swallow, eat a balanced diet, speak, smile, and go to school and work.
Treatments to fix these problems are expensive, so people of low socioeconomic means are less likely to receive quality care in a timely manner. Importantly, untreated gum disease is associated with serous systemic conditions such as diabetes, heart disease, and Alzheimer’s disease.
A person experiencing poverty also may be at increased risk for mental illness. That, in turn, can make it hard to practice oral hygiene, such as toothbrushing and flossing, or to maintain a relationship with a dental provider. Mental illnesses and substance use disorders often go hand-in-hand, and overuse of opioids, alcohol, and tobacco products also can raise the risk for tooth decay, gum disease, and oral cancers. Untreated dental diseases in this setting can cause pain, sometimes leading to increased substance use as a means of self-medication.
Research to understand better the connections between mental health, addiction, and oral health, particularly as they relate to health disparities, can help us develop more effective ways to treat patients. It also will help us prepare health providers, including dentists, to deliver the right kind of care to patients.
Another area that is ripe for investigation is to find ways to make it easier for people to get dental care, especially those from marginalized or rural communities. For example, the COVID-19 pandemic spurred more dentists to use teledentistry, where practitioners meet with patients remotely as a way to provide certain aspects of care, such as consultations, oral health screenings, treatment planning, and education.
Teledentistry holds promise as a cost-saving approach to connect dentists to people living in regions that may have a shortage of dentists. Some evidence suggests that providing access to oral health care outside of dental clinics—such as in schools, primary care offices, and community centers—has helped reduce oral health disparities in children. We need additional research to find out if this type of approach also might reduce disparities in adults.
These are just some of the opportunities highlighted in the Oral Health in America report that will inform NIDCR’s research in the coming years. Just as science, innovation, and new technologies have helped solve some of the most challenging health problems of our time, so too can they lead us to solutions for tackling oral health disparities. Our job will not be done until we can improve oral and overall health for everyone across America.
Links:
Oral Health in America: Advances and Challenges (National Institute of Dental and Craniofacial Research/NIH)
Oral Health in America Editors Issue Guidance for Improving Oral Health for All (NIDCR)
NIH, HHS Leaders Call for Research and Policy Changes To Address Oral Health Inequities (NIDCR)
NIH/NIDCR Releases Oral Health in America: Advances and Challenges (NIDCR)
Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 11th in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
10 Years of Protecting Public Health Through Tobacco Regulatory Research
Posted on by David M. Murray, Ph.D., NIH Office of Disease Prevention

“Kids are flocking to flavored, disposable e-cigarettes, study finds” – The Washington Post
“New ‘candy’ e-cigs catch fire after U.S. regulators stamp out Juul’s flavors” – Reuters
Headlines like these highlight a real challenge for people who want to protect kids from the harms of using tobacco products. While flavors, such as mint, menthol, watermelon, and apple pie are safe to consume in food products, inhaling them in tobacco products can be harmful and put the health of our kids at risk.+
A special kind of research is needed to help public health authorities keep up with the latest changes and trends in tobacco products. That includes studying how these flavored tobacco products are attractively marketed to children and how quickly many started using them.
In 2013, NIH and the Food and Drug Administration (FDA) launched a unique interagency partnership called the Tobacco Regulatory Science Program (TRSP), directed by Helen Meissner. It aims to reduce the public health impact of tobacco product use across the country. The NIH administers the research program through the Office of Disease Prevention (ODP), which I lead, to help inform FDA’s tobacco regulatory priorities.
This unique partnership also represents a new field of study called tobacco regulatory research. It informs proposed regulations for tobacco products based on strong scientific evidence. The TRSP brings together scientists from diverse fields, such as epidemiology, chemistry, toxicology, addiction, and psychology, to shed light on why people try and continue to use tobacco, how tobacco use affects health, and which policies might help reduce the risk of harm.
Now celebrating its 10th anniversary, this extremely productive partnership has resulted in more than 400 research grants, all peer-reviewed and designed to increase our understanding of existing and emerging tobacco products and their associated health risks.
Our research includes studies showing that menthol in cigarettes makes it easier to start smoking by reducing the harshness of tobacco [1]. People who smoke menthol cigarettes also show more signs of nicotine dependence and, therefore, are less likely to successfully quit. The research shows this is because menthol interacts with nicotine in the brain, making nicotine even more addictive.
Additionally, researchers have explored how marketing and promotion of menthol and flavored tobacco products have targeted Black and LGBTQ+ people, socioeconomically disadvantaged populations, and people with mental health challenges. These studies show that this direct marketing has contributed to the burden of tobacco-related disease among these groups and widened health inequities [2].
The TRSP also has a real-world impact on shaping tobacco policy. In April 2022, the program’s sponsored research was cited in FDA-proposed rules to prohibit menthol as a characterizing flavor in cigarettes and ban all characterizing flavors (other than tobacco) in cigars [3]. These tobacco product standards will have a huge impact on public health by reducing youth experimentation with products like cigarettes, cigars, and cigarillos and increasing the number of people who quit smoking.
Many jurisdictions have already banned flavored tobacco products. Through our partnership with the FDA, TRSP-funded researchers have started evaluating the impact of these policies on tobacco use and public health. The need for research continues as we seek to understand how new tobacco products affect people’s use, attitudes, and health.
However, tobacco products that have the potential to addict a new generation to nicotine continue to be marketed. For example, new products that use “ice-hybrid” flavors which combine cooling and fruity/sweet properties, such as raspberry ice, are being used more often than either fruity/sweet or menthol/mint among young adult e-cigarette users [4]. Illegally marketed, but novel, flavored oral nicotine products, such as gummies and pouches, also are gaining appeal among young people. The dynamic nature of the tobacco market emphasizes the importance of TRSP to support research on tobacco products, directly informing tobacco regulation.
The success of TRSP over the past 10 years demonstrates how establishing a research pipeline that directly informs regulation can lead to effective, evidence-based health policies. The high output of research on the effects of new and emerging tobacco products, such as the appeal and addictiveness of flavored e-cigarettes, provides FDA with data to inform regulatory actions. This partnership is truly helping regulators and policymakers turn scientific discovery into actions designed to protect public health.
References:
[1] Use of menthol cigarettes, smoking frequency, and nicotine dependence among US youth. Leas EC, Benmarhnia T, Strong DR, Pierce JP. JAMA Netw Open. 2022 Jun 1;5(6):e2217144.
[2] Menthol smoking and related health disparities. Centers for Disease Control and Prevention, June 27, 2022.
[3] FDA proposes rules prohibiting menthol cigarettes and flavored cigars to prevent youth initiation, significantly reduce tobacco-related disease and death. FDA News Release, April 28, 2022.
[4] ‘Ice’ flavoured e-cigarette use among young adults. Leventhal A, Dai H, Barrington-Trimis J, Sussman S. Tob Control. 2023 Jan;32(1):114-117.
Links:
Smokefree.gov (U.S. Department of Health and Human Services, Washington, D.C.)
Office of Disease Prevention (NIH)
Tobacco Regulatory Science Program (ODP)
Director’s Messages (ODP)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 29th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Help for Babies Born Dependent on Opioids
Posted on by Lawrence Tabak, D.D.S., Ph.D.

It’s been estimated that every 18 minutes in the United States, a newborn baby starts life with painful withdrawals from exposure to opioids in the womb. It’s called neonatal opioid withdrawal syndrome (NOWS), and it makes for a challenging start in life. These infants may show an array of withdrawal symptoms, including tremors, extreme irritability, and problems eating and sleeping.
Many of these infants experience long, difficult hospital stays to help them manage their withdrawal symptoms. But because hospital staff have no established evidence-based treatment standards to rely on, there is substantial variation in NOWS treatment around the country. There also are many open questions about the safest and most-effective way to support these babies and their families.
But answers are coming. The New England Journal of Medicine just published clinical trial results that evaluated care for infants with NOWS and which offer some much needed—and rather encouraging—data for families and practitioners [1]. The data are from the Eating, Sleeping, Consoling for Neonatal Opioid Withdrawal (ESC-NOW) trial, led by Leslie W. Young, The University of Vermont’s Larner College of Medicine, Burlington, and her colleagues Lori Devlin and Stephanie Merhar.
The ESC-NOW study is supported through the Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW) Collaborative. ACT NOW is an essential part of the NIH Helping to End Addiction Long-term (HEAL) Initiative, an aggressive effort to speed scientific solutions to stem the national opioid public health crisis and improve lives.
The latest study puts to the test two different approaches to care for newborns with NOWS. The first approach relies on the Finnegan Neonatal Abstinence Scoring Tool. For almost 50 years, doctors primarily assessed NOWS using this tool. It is based on a scoring system of 21 signs of withdrawal, including disturbances in a baby’s nervous system, metabolism, breathing, digestion, and more. However, there have been concerns that this scoring tool has led to an overreliance on treating babies with opioid medications, including morphine and methadone.
The other approach is known as Eat, Sleep, Console (ESC) care [2]. First proposed in 2014, ESC care has been adopted in many hospitals around the world. Rather than focusing on a long list of physical signs of withdrawal, this approach relies on a simpler functional assessment of whether an infant can eat, sleep, and be consoled. It emphasizes treatments other than medication, such as skin-to-skin contact, breastfeeding, and care from their mothers or other caregivers in a calm and nurturing environment.
The ESC care approach places an emphasis on the use of supportive interventions and aims to empower families in the care and nurturing of their infants. While smaller quality improvement studies of ESC have been compelling, the question at issue is whether the Eat, Sleep, Console care approach can reduce the time until infants with NOWS are medically ready to go home from the hospital in a wide variety of hospital settings—and, most importantly, whether it can do so safely.
To find out, the ESC-NOW team enrolled 1,305 infants with NOWS who were born after at least 36 weeks gestation. The study’s young participants were largely representative of infants with NOWS in the U.S., although non-Hispanic Black and Hispanic infants were slightly overrepresented. The babies were born at one of 26 U.S. hospitals, and each hospital was randomly assigned to transition from usual care using the Finnegan tool to the ESC care approach at a designated time.
Each hospital had a three-month transition period between the usual care and the ESC to allow clinical teams time to train on the new approach. The trial primarily aimed to understand if there was a significant difference in how long newborns with NOWS spent in the hospital before being medically ready for discharge between those receiving usual care versus those receiving ESC care. Researchers also assessed infants for safety, tracking both safety events that occurred during the hospital stay and events that occurred after the baby left the hospital, such as non-accidental trauma or death during an infant’s first three months.
The reported results reflect 837 of the 1,305 infants, who met the study definition of being medically ready for discharge. Infants who were discharged before meeting the study criteria, which were informed by the 2012 American Academy of Pediatrics recommendations for monitoring of infants with NOWS, were not included in the primary analysis.
Among the 837 infants, those who received ESC care were medically ready for discharge significantly sooner than those who received usual care. On average, they were medically ready to go home after about eight days compared to almost 15 days for the usual care group.
Many fewer infants in the ESC care group were treated with opioids compared to the usual care group (19.5 percent versus 52.0 percent). In more good news for ESC care, there was no difference in safety outcomes through the first three months despite the shorter hospital stays and reduced opioid treatment in the hospital. Infants who were cared for using the ESC care approach were no more likely to visit the doctor’s office, emergency room, or hospital after being discharged from the hospital.
More long-term study is needed to evaluate these children over months and years as they continue to develop and grow. Many of the infants in this study will be evaluated for the first two years of life to assess the long-term impact of ESC care on development and other outcomes. These findings offer encouraging early evidence that the ESC care approach is safe and effective. Although there was some variability in the outcomes, this study also shows that this approach can work well across diverse hospitals and communities.
The ESC-NOW trial is just one portion of the NIH Heal Initiative’s ACT NOW program, focused on gathering scientific evidence on how to care for babies with NOWS. Other studies are evaluating how to safely wean babies who do receive treatment with medication off opioids more quickly. The ACT NOW Longitudinal Study also will enroll at least 200 babies with prenatal opioid exposure and another 100 who were not exposed to better understand the long-term implications of early opioid exposure.
I’ve been anxious to see the results of the ESC-NOW study for a few months. It’s been worth the wait. The results show that we’re headed in the right direction with learning how best to treat NOWS and help to improve the lives of these young children and their families in the months and years ahead.
References:
[1] Eat, Sleep, Console Approach versus usual care for neonatal opioid withdrawal. Young LW, Ounpraseuth ST, Merhar SL, Newman S, Snowden JN, Devlin LA, et al. NEJM, 2023 Apr 30 [Published online ahead of print]
[2] An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Grossman MR, Berkwitt AK, Osborn RR, Xu Y, Esserman DA, Shapiro ED, Bizzarro MJ. Pediatrics. 2017 Jun;139(6):e20163360.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
“Eat, Sleep, Console” reduces hospital stay and need for medication among opioid-exposed infants, NIH news release, May 1, 2023
Helping to End Addiction Long-term® (HEAL) Initiative (NIH)
Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW)
Environmental Influences on Child Health Outcomes (ECHO) Program (NIH)
Leslie Young (The University of Vermont, Larner College of Medicine, Burlington)
NIH Support: The Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Center for Advancing Translational Sciences; Office of the Director
NIH HEAL Initiative Meets People Where They Are
Posted on by Rebecca Baker, Ph.D., NIH Helping to End Addiction Long-term® (HEAL) Initiative

The opioid crisis continues to devastate communities across America. Dangerous synthetic opioids, like fentanyl, have flooded the illicit drug supply with terrible consequences. Tragically, based on our most-recent data, about 108,000 people in the U.S. die per year from overdoses of opioids or stimulants [1]. Although this complex public health challenge started from our inability to treat pain effectively, chronic pain remains a life-altering problem for 50 million Americans.
To match the size and complexity of the crisis, in 2018 NIH developed the NIH Helping to End Addiction Long-term® (HEAL) Initiative, an aggressive effort involving nearly all of its 27 institutes and centers. Through more than 1,000 research projects, including basic science, clinical testing of new and repurposed drugs, research with communities, and health equity research, HEAL is dedicated to building a new future built on hope.
In this future:
- A predictive tool used during a health visit personalizes treatment for back pain. The tool estimates the probability that a person will benefit from physical therapy, psychotherapy, or surgery.
- Visits to community health clinics and emergency departments serve as routine opportunities to prevent and treat opioid addiction.
- Qualified school staff and pediatricians screen all children for behavioral and other mental health conditions that increase risk for harmful developmental outcomes, including opioid misuse.
- Infants born exposed to opioids during a mother’s pregnancy receive high-quality care—setting them up for a healthy future.
Five years after getting started (and interrupted by a global pandemic), HEAL research is making progress toward achieving this vision. I’ll highlight three ways in which scientific solutions are meeting people where they are today.
A Window of Opportunity for Treatment in the Justice System
Sadly, jails and prisons are “ground zero” for the nation’s opioid crisis. Eighty-five percent of people who are incarcerated have a substance use disorder or a history of substance use. Our vision at HEAL is that every person in jail, prison, or a court-supervised program receives medical care, which includes effective opioid use disorder treatment.
Some research results already are in supporting this approach: A recent HEAL study learned that individuals who had received addiction treatment while in one Massachusetts jail were about 30 percent less likely to be arrested, arraigned, or incarcerated again compared with those incarcerated during the same time period in a neighboring jail that did not offer treatment [2]. Research from the HEAL-supported Justice Community Opioid Innovation Network also is exploring public perceptions about opioid addiction. One such survey showed that most U.S. adults see opioid use disorder as a treatable medical condition rather than as a criminal matter [3]. That’s hopeful news for the future.
A Personalized Treatment Plan for Chronic Back Pain
Half of American adults live with chronic back pain, a major contributor to opioid use. The HEAL-supported Back Pain Consortium (BACPAC) is creating a whole-system model for comprehensive testing of everything that contributes to chronic low back pain, from anxiety to tissue damage. It also includes comprehensive testing of promising pain-management approaches, including psychotherapy, antidepressants, or surgery.
Refining this whole-system model, which is nearing completion, includes finding computer-friendly ways to describe the relationship between the different elements of pain and treatment. That might include developing mathematical equations that describe the physical movements and connections of the vertebrae, discs, and tendons.
Or it might include an artificial intelligence technique called machine learning, in which a computer looks for patterns in existing data, such as electronic health records or medical images. In keeping with HEAL’s all-hands-on-deck approach, BACPAC also conducts clinical trials to test new (or repurposed) treatments and develop new technologies focused on back pain, like a “wearable muscle” to help support the back.
Harnessing Innovation from the Private Sector
The HEAL research portfolio spans basic science to health services research. That allows us to put many shots on goal that will need to be commercialized to help people. Through its research support of small businesses, HEAL funding offers a make-or-break opportunity to advance a great idea to the marketplace, providing a bridge to venture capital or other larger funding sources needed for commercialization.
This bridge also allows HEAL to invest directly in the heart of innovation. Currently, HEAL funds nearly 100 such companies across 20 states. While this is a relatively small portion of all HEAL research, it is science that will make a difference in our communities, and these researchers are passionate about what they do to build a better future.
A couple of current examples of this research passion include: delivery of controlled amounts of non-opioid pain medications after surgery using a naturally absorbable film or a bone glue; immersive virtual reality to help people with opioid use disorder visualize the consequences of certain personal choices; and mobile apps that support recovery, taking medications, or sensing an overdose.
In 2023, HEAL is making headway toward its mission to accelerate development of safe, non-addictive, and effective strategies to prevent and treat pain, opioid misuse, and overdose. We have 314 clinical trials underway and 41 submissions to the Food and Drug Administration to begin clinical testing of investigational new drugs or devices: That number has doubled in the last year. More than 100 projects alone are addressing back pain, and more than 200 projects are studying medications for opioid use disorder.
The nation’s opioid crisis is profoundly difficult and multifaceted—and it won’t be solved with any single approach. Our research is laser-focused on its vision of ending addiction long-term, including improving pain management and expanding access to underused, but highly effective, addiction medications. Every day, we imagine a better future for people with physical and emotional pain and communities that are hurting. Hundreds of researchers and community members across the country are working to achieve a future where people and communities have the tools they need to thrive.
References:
[1] Provisional drug overdose death counts. Ahmad FB, Cisewski JA, Rossen LM, Sutton P. National Center for Health Statistics. 2023.
[2] Recidivism and mortality after in-jail buprenorphine treatment for opioid use disorder. Evans EA, Wilson D, Friedmann PD. Drug Alcohol Depend. 2022 Feb 1;231:109254.
[3] Social stigma toward persons with opioid use disorder: Results from a nationally representative survey of U.S. adults. Taylor BG, Lamuda PA, Flanagan E, Watts E, Pollack H, Schneider J. Subst Use Misuse. 2021;56(12):1752-1764.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
NIH Helping to End Addiction Long-term® (HEAL) Initiative
Video: The NIH HEAL Initiative–HEAL Is Hope
Justice Community Opioid Innovation Network (HEAL)
Back Pain Consortium Research Program (HEAL)
NIH HEAL Initiative 2023 Annual Report (HEAL)
Small Business Programs (HEAL)
Rebecca Baker (HEAL)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 28th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Updating an Rx for Progress
Posted on by Lawrence Tabak, D.D.S., Ph.D.

On April 11, I took part in an afternoon plenary session titled: “State of the Science: Updates from the National Institutes of Health.” Joining me on the stage were my NIH colleagues Nora Volkow, director, National Institute on Drug Abuse; and George Koob, director, National Institute on Alcohol Abuse and Alcoholism. I was able to update everyone on the research progress being made by the NIH Helping to End Addiction (HEAL) Initiative. The initiative, through its support of more than 1,000 projects across the nation, aims to prevent addiction through enhanced pain management, while seeking better ways to improve prevention and treatment for opioid misuse disorder and addiction. The Rx and Illicit Drug Summit 2023 took place on April 11-13 at the Georgia World Congress Center. Credit: Pierce Harman, HMP Global, Malvern, PA.
Changes in Normal Brain Connections Linked to Eating Disorders
Posted on by Lawrence Tabak, D.D.S., Ph.D.
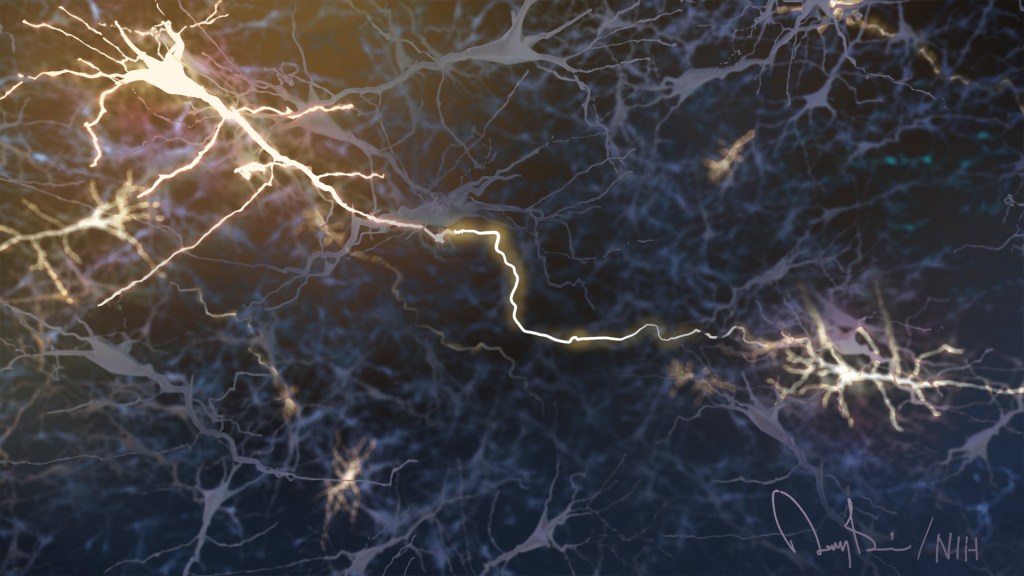
Anyone who has ever had a bad habit knows how vexingly difficult breaking it can be. The reason is the repeated action, initially linked to some type of real or perceived reward, over time changes the way our very brains are wired to work. The bad habit becomes automatic, even when the action does us harm or we no longer wish to do it.
Now an intriguing new study shows that the same bundled nerve fibers, or brain circuits, involved in habit formation also can go awry in people with eating disorders. The findings may help to explain why eating disorders are so often resistant to will power alone. They also may help to point the way to improved approaches to treating eating disorders, suggesting strategies that adjust the actual brain circuitry in helpful ways.
These latest findings, published in the journal Science Translational Medicine, come from the NIH-supported Casey Halpern, University of Pennsylvania’s Perelman School of Medicine, Philadelphia, and Cara Bohon, Stanford University School of Medicine, Stanford, CA [1].
Halpern, Bohon, and colleagues were interested in a growing body of evidence linking habitual behaviors to mental health conditions, most notably substance use disorders and addictions. But what especially intrigued them was recent evidence also suggesting a possible role for habitual behaviors in the emergence of eating disorders.
To look deeper into the complex circuitry underlying habit formation and any changes there that might be associated with eating disorders, they took advantage of a vast collection of data from the NIH-funded Human Connectome Project (HCP). It was completed several years ago and now serves as a valuable online resource for researchers.
The HCP offers a detailed wiring map of a normal human brain. It describes all the structural and functional neural connections based on careful analyses of hundreds of high-resolution brain scans. These connections are then layered with genetic, behavioral, and other types of data. This incredible map now allows researchers to explore and sometimes uncover the roots of neurological and mental health conditions within the brain’s many trillions of connections.
In the new study, Halpern, Bohon, and colleagues did just that. First, they used sophisticated mapping methods in 178 brain scans from the HCP data to locate key portions of a brain region called the striatum, which is thought to be involved in habit formation. What they really wanted to know was whether circuits operating within the striatum were altered in some way in people with binge eating disorder or bulimia nervosa.
To find out, the researchers recruited 34 women who have an eating disorder and, with their consent, imaged their brains using a variety of techniques. Twenty-one participants were diagnosed with binge eating disorder, and 13 had bulimia nervosa. For comparison purposes, the researchers looked at the same brain circuits in 19 healthy volunteers.
The two groups were otherwise similar in terms of their ages, weights, and other features. But the researchers suspected they might find differences between the healthy group and those with an eating disorder in brain circuits known to have links to habitual behaviors. And, indeed, they did.
In comparison to a “typical” brain, those from people with an eating disorder showed striking changes in the connectivity of a portion of the striatum known as the putamen. That’s especially notable because the putamen is known for its role in learning and movement control, including reward, thinking, and addiction. What’s more, those observed changes in the brain’s connections and circuitry in this key brain area were more evident in people whose eating disorder symptoms and emotional eating were more frequent and severe.
Using other brain imaging methods in 10 of the volunteers (eight with binge eating disorder and two healthy controls), the researchers also connected those changes in the habit-forming brain circuits to high levels of a protein receptor that responds to dopamine. Dopamine is an important chemical messenger in the brain involved in pleasure, motivation, and learning. They also observed in those with eating disorders structural changes in the architecture of the densely folded, outer layer of the brain known as grey matter.
While there’s much more to learn, the researchers note the findings may lead to future treatments aimed to modify the brain circuitry in beneficial ways. Indeed, Halpern already has encouraging early results from a small NIH-funded clinical trial testing the ability of deep brain stimulation (DBS) in people with binge eating disorder to disrupt signals that drive food cravings in another portion of the brain associated with reward and motivation, known as the nucleus accumbens, [2]. In DBS, doctors implant a pacemaker-like device capable of delivering harmless therapeutic electrical impulses deep into the brain, aiming for the spot where they can reset the abnormal circuitry that’s driving eating disorders or other troubling symptoms or behaviors.
But the latest findings published in Science Translational Medicine now suggest other mapped brain circuits as potentially beneficial DBS targets for tackling binge eating, bulimia nervosa, or other life-altering, hard-to-treat eating disorders. They also may ultimately have implications for treating other conditions involving various other forms of compulsive behavior.
These findings should come as a source of hope for the family and friends of the millions of Americans—many of them young people—who struggle with eating disorders. The findings also serve as an important reminder for the rest of us that, despite common misconceptions that disordered eating is a lifestyle choice, these conditions are in fact complex and serious mental health problems driven by fundamental changes in the brain’s underlying circuitry.
Finding new and more effective ways to treat serious eating disorders and other compulsive behaviors is a must. It will require equally serious ongoing efforts to unravel their underlying causes and find ways to alter their course—and this new study is an encouraging step in that direction.
References:
[1] Human habit neural circuitry may be perturbed in eating disorders. Wang AR, Kuijper FM, Barbosa DAN, Hagan KE, Lee E, Tong E, Choi EY, McNab JA, Bohon C, Halpern CH. Sci Transl Med. 2023 Mar 29;15(689):eabo4919.
[2] Pilot study of responsive nucleus accumbens deep brain stimulation for loss-of-control eating. Shivacharan RS, Rolle CE, Barbosa DAN, Cunningham TN, Feng A, Johnson ND, Safer DL, Bohon C, Keller C, Buch VP, Parker JJ, Azagury DE, Tass PA, Bhati MT, Malenka RC, Lock JD, Halpern CH. Nat Med. 2022 Sep;28(9):1791-1796.
Links:
Eating Disorders (National Institute of Mental Health/NIH)
Casey Halpern (Penn Medicine, Philadelphia)
Cara Bohon (Stanford University, Stanford, CA)
NIH Support: National Institute of Mental Health; National Institute of Neurological Disorders and Stroke
Tackling Complex Scientific Questions Requires a Team Approach
Posted on by Nora D. Volkow, M.D., National Institute on Drug Abuse

During the COVID-19 pandemic, we have seen unprecedented, rapid scientific collaboration, as experts around the world in discrete, previously disconnected fields, have found ways to collaborate to face a common cause. For example, physicists helped respiratory specialists understand how virus particles could spread in air, leading to improved mitigation strategies. Specialists in cardiovascular science, neuroscience, immunology, and other fields are now working together to understand and address Long COVID. Over the past two years, we have also seen remarkable international sharing of epidemiological data and information on effects of vaccines.
Science is increasingly a team activity, which is true for many fields, not just biomedicine. The professional diversity of research teams reflects the increased complexity of the questions science is called upon to answer. This is especially obvious in the study of the brain, which is the most complex system known to us.
The NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, with the goal of vastly enhancing neuroscience through new technologies, includes research teams with neuroscientists, engineers, mathematicians, physicists, data scientists, ethicists, and more. Nearly half (47 percent) of grant awards have multiple principal investigators.
Besides the BRAIN Initiative, other multi-institute NIH research projects are applying team science to complex research questions, such as those related to neurodevelopment, addiction, and pain. The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, created a team-based research framework to advance promising pain therapeutics quickly to clinical testing.
In the Adolescent Brain Cognitive Development (ABCD) study, which is led by NIDA in close partnership with NIH’s National Institute on Alcohol Abuse and Alcoholism (NIAAA), and other NIH institutes, 21 research centers are collecting behavioral, biospecimen, and neuroimaging data from 11,878 children from age 10 through their teens. Teams led by experts in adolescent psychiatry, developmental psychology, and pediatrics interview participants and their families. These experts then gather a battery of health metrics from psychological, cognitive, sociocultural, and physical assessments, including collection and analysis of various kinds of biospecimens (blood, saliva). Further, experts in biophysics gather information on the structure and function of participants’ brains every two years.
A similar study of young children in the first decade of life beginning with the prenatal period, the HEALthy Brain and Child Development (HBCD) study, supported by HEAL, NIDA, and several other NIH institutes and centers, is now underway at 25 research sites across the country. A range of scientific specialists, similar to that in the ABCD study, is involved in this effort. In this case, they are aided by experts in obstetric care and in infant neuroimaging.
For both of these studies, teams of data scientists validate and curate all the information generated and make it available to researchers across the world. This makes it possible to investigate complex questions such as human neurodevelopmental diversity and the effects of genes and social experiences and their relation to mental health. More than half of the publications using ABCD data have been authored by non-ABCD investigators taking advantage of the open-access format.
Yet, institutions that conduct and fund science—including NIH—have been slow to support and reward collaboration. Because authorship and funding are so important in tenure and promotion decisions at universities, for example, an individual’s contribution to larger, multi-investigator projects on which they may not be the grantee or lead author on a study publication may carry less weight.
For this reason, early-career scientists may be particularly reluctant to collaborate on team projects. Among the recommendations of a 2015 National Academies of Sciences, Engineering, and Medicine (NASEM) report, Enhancing the Effectiveness of Team Science, was that universities and other institutions should find effective ways to give credit for team-based work to assist promotion and tenure committees.
The strongest teams will be diverse in other respects, not just scientific expertise. Besides more actively fostering productive collaborations across disciplines, NIH is making a more concerted effort to promote racial equity and inclusivity in our research workforce, both through the NIH UNITE Initiative and through Institute-specific initiatives like NIDA’s Racial Equity Initiative.
To promote diversity, inclusivity, and accessibility in research, the BRAIN Initiative recently added a requirement in most of its funding opportunity announcements (FOAs) that has applicants include a Plan for Enhancing Diverse Perspectives (PEDP) in the proposed research. The PEDPs are evaluated and scored during the peer review as part of the holistic considerations used to inform funding decisions. These long-overdue measures will not only ensure that NIH-funded science is more diverse, but they are also important steps toward studying and addressing social determinants of health and the health disparities that exist for so many conditions.
Increasingly, scientific discovery is as much about exploring new connections between different kinds of researchers as it is about finding new relationships among different kinds of scientific databases. The challenges before us are great—ending the COVID pandemic, finding a solution to the addiction and overdose crisis, and so many others—and increased collaboration between scientists will give us the greatest chance to successfully overcome these challenges.
Links:
Nora Volkow’s Blog (National Institute on Drug Abuse/NIH)
Adolescent Brain Cognitive Development Study
Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Racial Equity Initiative (NIDA)
Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 13th in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
Small Study Suggests Approved Insomnia Drug Can Aid in Opioid Recovery
Posted on by Lawrence Tabak, D.D.S., Ph.D.

Opioid use disorders (OUD) now threaten the health and lives of far too many young and adult Americans. While getting treatment is a key first step to recovery, overcoming an opioid addiction often comes with brutal withdrawal symptoms, including bad bouts of insomnia that are often untreatable with traditional prescription sleep medications. These medications act as sedatives, making them unsafe for people in OUD recovery.
But now, researchers have found that an approved drug for insomnia that works differently than other sleep medications could offer some needed help for the sleeplessness that affects those overcoming an opioid addiction [1]. The drug, known as suvorexant (Belsomra ®), was provided in a study to people during and immediately after tapering off opioids, and it allowed them to sleep significantly more during this week-long period. Suvorexant also helped to reduce their opioid withdrawal and craving.
This study, which received support from NIH’s Helping to End Addiction Long-term (HEAL) Initiative certainly offers promising news. The Food and Drug Administration (FDA) approved suvorexant to treat insomnia in 2014, and it is available for off-label use to help people overcoming an OUD.
The good news, however, comes with a major caveat. This early clinical trial had relatively small enrollment numbers, and larger studies are definitely needed to follow up and confirm the initial results.
The latest findings, published in the journal Science Translational Medicine, come from a team at Johns Hopkins University School of Medicine, Baltimore, led by Andrew Huhn. He and colleagues recognized sleep disturbances as a severe problem during recovery. They wondered whether suvorexant might help.
Suvorexant doesn’t actively sedate people like other sleeping medications. Suvorexant works by targeting orexin, a biochemical made in the brain that helps keep you awake [2]. Interestingly, orexin signals also have been implicated in opioid withdrawal symptoms, sleep disturbances, and drug-seeking behaviors.
Thirty-eight people entered the Hopkins study, and 26 completed it. Their average age was about 40, with close to equal numbers of white and Black participants. Most were male, and all were undergoing supervised withdrawal treatment with buprenorphine/naloxone, which is used in combination as a medication-assisted treatment for OUD.
To find out if suvorexant helped, the researchers measured total sleep time nightly using wireless devices that recorded brain activity and movement in people taking either 20 milligrams or 40 milligrams of suvorexant versus a placebo. The researchers also used standard methods to assess symptoms of opioid withdrawal, along with suvorexant’s potential for abuse.
The data showed that people taking suvorexant over four days while tapering off opioids slept about 90 minutes longer per night on average. They also continued to sleep for an extra hour a night on average in the four days following the tapering period. The researchers note that these increases in sleep duration far exceed the American Academy of Sleep Medicine’s threshold for clinically meaningful improvement.
The researchers also didn’t see any differences in adverse events between those taking suvorexant versus a placebo. They also note that the main side effect of suvorexant in general is feeling sleepy the next day as the drug wears off slowly. There also wasn’t any evidence that suvorexant might come with a risk for drug abuse.
However, because the study was small, it lacked the needed statistical power to determine meaningful differences between the two doses of suvorexant. The study also didn’t include many women. But overall, the evidence that suvorexant or even other medications that target orexin could improve OUD treatment appears quite promising.
The NIH’s HEAL Initiative has launched over 600 research projects across the country. These studies cover a range of science and health care needs. But a common thread running through these projects is a desire to enhance the evidence base for lifesaving OUD interventions. Another is a commitment to discover better ways to help people recover from an OUD, and these latest data on suvorexant show this commitment in action.
References:
[1] Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper. Huhn AS, Finan PH, Gamaldo CE, Hammond AS, Umbricht A, Bergeria CL, Strain EC, Dunn KE. Sci Transl Med. 2022 Jun 22;14(650):eabn8238.
[2] The hypocretin/orexin system. Ebrahim IO, et al. J R Soc Med. 2002 May;95(5):227-30.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
Opioids (National Institute on Drug Abuse/NIH)
Helping to End Addiction Long-term (HEAL) Initiative (NIH)
Andrew Huhn (Johns Hopkins School of Medicine, Baltimore)
NIH Support: National Institute on Drug Abuse
Research to Address the Real-Life Challenges of Opioid Crisis
Posted on by Lawrence Tabak, D.D.S., Ph.D.

While great progress has been made in controlling the COVID-19 pandemic, America’s opioid crisis continues to evolve in unexpected ways. The opioid crisis, which worsened during the pandemic and now involves the scourge of fentanyl, claims more than 70,000 lives each year in the United States [1]. But throughout the pandemic, NIH has continued its research efforts to help people with a substance use disorder find the help that they so need. These efforts include helping to find relief for the millions of Americans who live with severe and chronic pain.
Recently, I traveled to Atlanta for the Rx and Illicit Drug Summit 2022. While there, I moderated an evening fireside chat with two of NIH’s leaders in combating the opioid crisis: Nora Volkow, director of the National Institute on Drug Abuse (NIDA); and Rebecca Baker, director of Helping to End Addiction Long-term® (HEAL) initiative. What follows is an edited, condensed transcript of our conversation.
Tabak: Let’s start with Nora. When did the opioid crisis begin, and how has it changed over the years
Volkow: It started just before the year 2000 with the over-prescription of opioid medications. People were becoming addicted to them, many from diverted product. By 2010, CDC developed guidelines that decreased the over-prescription. But then, we saw a surge in heroin use. That turned the opioid crisis into two problems: prescription opioids and heroin.
In 2016, we encountered the worst scourge yet. It is fentanyl, an opioid that’s 50 times more potent than heroin. Fentanyl is easily manufactured, and it’s easier than other opioids to hide and transport across the border. That makes this drug very profitable.
What we have seen during the pandemic is the expansion of fentanyl use in the United States. Initially, fentanyl made its way to the Northeast; now it’s everywhere. Initially, it was used to contaminate heroin; now it’s used to contaminate cocaine, methamphetamine, and, most recently, illicit prescription drugs, such as benzodiazepines and stimulants. With fentanyl contaminating all these drugs, we’re also seeing a steep rise in mortality from cocaine and methamphetamine use in African Americans, American Indians, and Alaska natives.
Tabak: What about teens? A recent study in the journal JAMA reported for the first time in a decade that overdose deaths among U.S. teens rose dramatically in 2020 and kept rising through 2021 [2]. Is fentanyl behind this alarming increase?
Volkow: Yes, and it has us very concerned. The increase also surprised us. Over the past decade, we have seen a consistent decrease in adolescent drug use. In fact, there are some drugs that have the lowest usage rates that we’ve ever recorded. To observe this more than doubling of overdose deaths from fentanyl before the COVID pandemic was a major surprise.
Adolescents don’t typically use heroin, nor do they seek out fentanyl. Our fear is adolescents are misusing illicit prescriptions contaminated with fentanyl. Because an estimated 30-40 percent of those tainted pills contain levels of fentanyl that can kill you, it becomes a game of Russian roulette. This dangerous game is being played by adolescents who may just be experimenting with illicit pills.
Tabak: For people with substance use disorders, there are new ways to get help. In fact, one of the very few positive outcomes of the pandemic is the emergence of telehealth. If we can learn to navigate the various regulatory issues, do you see a place for telehealth going forward?
Volkow: When you have a crisis like this one, there’s a real need to accelerate interventions and innovation like telehealth. It certainly existed before the pandemic, and we knew that telehealth was beneficial for the treatment of substance use disorders. But it was very difficult to get reimbursement, making access extremely limited.
When COVID overwhelmed emergency departments, people with substance use disorders could no longer get help there. Other interventions were needed, and telehealth helped fill the void. It also had the advantage of reaching rural populations in states such as Kentucky, West Virginia, Ohio, where easy access to treatment or unique interventions can be challenging. In many prisons and jails, administrators worried about bringing web-based technologies into their facilities. So, in partnership with the Justice Department, we have created networks that now will enable the entry of telehealth into jails and prisons.
Tabak: Rebecca, it’s been four years since the HEAL initiative was announced at this very summit in 2018. How is the initiative addressing this ever-evolving crisis?
Baker: We’ve launched over 600 research projects across the country at institutions, hospitals, and research centers in a broad range of scientific areas. We’re working to come up with new treatment options for pain and addiction. There’s exciting research underway to address the craving and sleep disruption caused by opioid withdrawal. This research has led to over 20 investigational new drug applications to the FDA. Some are for repurposed drugs, compounds that have already been shown to be safe and effective for treating other health conditions that may also have value for treating addiction. Some are completely novel. We have also initiated the first testing of an opioid vaccine, for oxycodone, to prevent relapse and overdose in high-risk individuals.
Tabak: What about clinical research?
Baker: We’re testing multiple different treatments for both pain and addiction. Not everyone with pain is the same, and not every treatment is going to work the same for everyone. We’re conducting clinical trials in real-world settings to find out what works best for patients. We’re also working to implement lifesaving, evidence-based interventions into places where people seek help, including faith, community, and criminal justice settings.
Tabak: The pandemic highlighted inequities in our health-care system. These inequities afflict individuals and populations who are struggling with addiction and overdose. Nora, what needs to be done to address the social determinants of racial disparities?
Volkow: This is an extraordinarily important question. As you noted, certain racial and ethnic groups had disproportionately higher mortality rates from COVID. We have seen the same with overdose deaths. For example, we know that the most important intervention for preventing overdoses is to initiate medications such as methadone, buprenorphine or vivitrol. But Black Americans are initiated on these medications at least five years later than white Americans. Similarly, Black Americans also are less likely to receive the overdose-reversal medication naloxone.
That’s not right. We must ask what are the core causes of limited access to high-quality health care? Low income is a major contributing factor. Helping people get an education is one of the most important factors to address it. Another factor is distrust of the medical system. When racial and ethnic discrimination is compounded by discrimination because a person has a substance use disorder, you can see why it becomes very difficult for some to seek help. As a society, we certainly need to address racial discrimination. But we also need to address discrimination against substance use disorders in people of all races who are vulnerable.
Baker: Our research is tackling these barriers head on with a direct focus on stigma. As Nora alluded to, oftentimes providers may not offer lifesaving medication to some patients, and we’ve developed and are testing research training to help providers recognize and address their own biases and behaviors in caring for different populations.
We have supported research on the drivers of equity. A big part of this is engaging with people with lived experience and making sure that the interventions being designed are feasible in the real world. Not everyone has access to health insurance, transportation, childcare—the support that they may need to sustain treatment and recovery. In short, our research is seeking ways to enhance linkage to treatment.
Nora mentioned the importance of telehealth in improving equity. That’s another research focus, as well as developing tailored, culturally appropriate interventions for addressing pain and addiction. When you have this trust issue, you can’t always go in with a prescription or a recommendation from a physician. So in American and Alaskan native communities, we’re integrating evidence-based prevention approaches with traditional practices like wellness gatherings, cooking together, use of sage and spirituality, along with community support, and seeing if that encourages and increases the uptake of these prevention approaches in communities that need it so much.
Tabak: The most heartbreaking impact of the opioid crisis has been the infants born dependent on opioids. Rebecca, what’s being done to help the very youngest victims of the opioid crisis born with neonatal opioid withdrawal syndrome, or NOWS?
Baker: Thanks for asking about the infants. Babies with NOWS undergo withdrawal at birth and cry inconsolably, often with extreme stomach upset and sometimes even with seizures. Our research found that hospitals across the country vary greatly in how they treat these babies. Our program, ACT NOW, or Advancing Clinical Trials in Neonatal Opioid Withdrawal, aims to provide concrete guidance for nurses in the NICU treating these infants. One of the studies that we call Eat, Sleep, Console focuses on the abilities of the baby. Our researchers are testing if the ability to eat, sleep, or be consoled increases bonding with the mother and if it reduces time in the hospital, as well as other long-term health outcomes.
In addition to that NOWS program, we’ve also launched the HEALthy Brain and Child Development Study, or HBCD, that seeks to understand the long-term consequences of opioid exposure together with all the other environmental and other factors the baby experiences as they grow up. The hope is that together these studies will inform future prevention and treatment efforts for both mental health and also substance use and addiction.
Tabak: As the surge in heroin use and appearance of fentanyl has taught us, the opioid crisis has ever-changing dynamics. It tells us that we need better prevention strategies. Rebecca, could you share what HEAL is doing about prevention?
Baker: Prevention has always been a core component of the HEAL Initiative in a number of ways. The first is by preventing unnecessary opioid exposures through enhanced and evidence-based pain management. HEAL is supporting research on new small molecules, new devices, new biologic therapeutics that could treat pain and distinct pain conditions without opioids. And we’re also researching and providing guidance for clinicians on strategies for managing pain without medication, including acupuncture and physical therapy. They can often be just as effective and more sustainable.
HEAL is also working to address risky opioid use outside of pain management, especially in high-risk groups. That includes teens and young adults who may be experimenting, people lacking stable housing, patients who are on high-dose opioids for pain management, or they maybe have gone off high-dose opioids but still have them in their possession.
Finally, to prevent overdose we have to give naloxone to the people who need it. The HEALing Communities Study has taken some really innovative approaches to providing naloxone in libraries, on the beach, and places where overdoses are actually happening, not just in medical settings. And I think that will be, in our fight against the overdose crisis, a key tool.
Volkow: Larry, I’d like to add a few words on prevention. There are evidence-based interventions that have been shown to be quite effective for preventing substance use among teenagers and young adults. And yet, they are not implemented. We have evidence-based interventions that work for prevention. We have evidence-based interventions that work for treatment. But we don’t provide the resources for their implementation, nor do we train the personnel that can carry it over.
Science can give us tools, but if we do not partner at the next level for their implementation, those tools do not have the impact they should have. That’s why I always bring up the importance of policy in the implementation phase.
Tabak: Rebecca, the opioid crisis got started with a lack of good options for treating pain. Could you share with us how HEAL’s research efforts are addressing the needs of millions of Americans who experience both chronic pain and opioid use disorder?
Baker: It’s so important to remember people with pain. We can’t let our efforts to combat the opioid crisis make us lose sight of the needs of the millions of Americans with pain. One hundred million Americans experience pain; half of them have severe pain, daily pain, and 20 million have such severe pain that they can’t do things that are important to them in their life, family, job, other activities that bring their life meaning.
HEAL recognizes that these individuals need better options. New non-addictive pain treatments. But as you say, there is a special need for people with a substance use disorder who also have pain. They desperately need new and better options. And so we recently, through the HEAL Initiative, launched a new trials network that couples medication-based treatment for opioid use disorders, so that’s methadone or buprenorphine, with new pain-management strategies such as psychotherapy or yoga in the opioid use disorder treatment setting so that you’re not sending them around to lots of different places. And our hope is that this integrated approach will address some of the fragmented healthcare challenges that often results in poor care for these patients.
My last point would be that some patients need opioids to function. We can’t forget as we make sure that we are limiting risky opioid use that we don’t take away necessary opioids for these patients, and so our future research will incorporate ways of making sure that they receive needed treatment while also preventing them from the risks of opioid use disorder.
Tabak: Rebecca, let me ask you one more question. What do you want the folks here to remember about HEAL?
Baker: HEAL stands for Helping to End Addiction Long-term, and nobody knows more than the people in this room how challenging and important that really is. We’ve heard a little bit about the great promise of our research and some of the advances that are coming through our research pipeline, new treatments, new guidance for clinicians and caregivers. I want everyone to know that we want to work with you. By working together, I’m confident that we will tailor these new advances to meet the individual needs of the patients and populations that we serve.
Tabak: Nora, what would you like to add?
Volkow: This afternoon, I met with two parents who told me the story of how they lost their daughter to an overdose. They showed me pictures of this fantastic girl, along with her drawings. Whenever we think about overdose deaths in America, the sheer number—75,000—can make us indifferent. But when you can focus on one person and feel the love surrounding that life, you remember the value of this work.
Like in COVID, substance use disorders are a painful problem that we’re all experiencing in some way. They may have upset our lives. But they may have brought us together and, in many instances, brought out the best that humans can do. The best, to me, is caring for one another and taking the responsibility of helping those that are most vulnerable. I believe that science has a purpose. And here we have a purpose: to use science to bring solutions that can prevent and treat those suffering from substance use disorders.
Tabak: Thanks to both of you for this enlightening conversation.
References:
[1] Drug overdose deaths, Centers for Disease Control and Prevention, February 22, 2022.
[2] Trends in drug overdose deaths among US adolescents, January 2010 to June 2021. Friedman J. et al. JAMA. 2022 Apr 12;327(14):1398-1400.
Links:
Video: Evening Plenary with NIH’s Lawrence Tabak, Nora Volkow, and Rebecca Baker (Rx and Illicit Drug Summit 2022)
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
Opioids (National Institute on Drug Abuse/NIH)
Fentanyl (NIDA)
Helping to End Addiction Long-term®(HEAL) Initiative (NIH)
Rebecca Baker (HEAL/NIH)
Nora Volkow (NIDA)
NIH’s Nobel Winners Demonstrate Value of Basic Research
Posted on by Dr. Francis Collins

Last week was a big one for both NIH and me. Not only did I announce my plans to step down as NIH Director by year’s end to return to my lab full-time, I was reminded by the announcement of the 2021 Nobel Prizes of what an honor it is to be affiliated an institution with such a strong, sustained commitment to supporting basic science.
This year, NIH’s Nobel excitement started in the early morning hours of October 4, when two NIH-supported neuroscientists in California received word from Sweden that they had won the Nobel Prize in Physiology or Medicine. One “wake up” call went to David Julius, University of California, San Francisco (UCSF), who was recognized for his groundbreaking discovery of the first protein receptor that controls thermosensation, the body’s perception of temperature. The other went to his long-time collaborator, Ardem Patapoutian, Scripps Research Institute, La Jolla, CA, for his seminal work that identified the first protein receptor that controls our sense of touch.
But the good news didn’t stop there. On October 6, the 2021 Nobel Prize in Chemistry was awarded to NIH-funded chemist David W.C. MacMillan of Princeton University, N.J., who shared the honor with Benjamin List of Germany’s Max Planck Institute. (List also received NIH support early in his career.)
The two researchers were recognized for developing an ingenious tool that enables the cost-efficient construction of “greener” molecules with broad applications across science and industry—including for drug design and development.
Then, to turn this into a true 2021 Nobel Prize “hat trick” for NIH, we learned on October 12 that two of this year’s three Nobel winners in Economic Sciences had been funded by NIH. David Card, an NIH-supported researcher at University of California, Berkley, was recognized “for his empirical contributions to labor economics.” He shared the 2021 prize with NIH grantee Joshua Angrist of Massachusetts Institute of Technology, Cambridge, and his colleague Guido Imbens of Stanford University, Palo Alto, CA, “for their methodological contributions to the analysis of causal relationships.” What a year!
The achievements of these and NIH’s 163 past Nobel Prize winners stand as a testament to the importance of our agency’s long and robust history of investing in basic biomedical research. In this area of research, scientists ask fundamental questions about how life works. The answers they uncover help us to understand the principles, mechanisms, and processes that underlie living organisms, including the human body in sickness and health.
What’s more, each advance builds upon past discoveries, often in unexpected ways and sometimes taking years or even decades before they can be translated into practical results. Recent examples of life-saving breakthroughs that have been built upon years of fundamental biomedical research include the mRNA vaccines for COVID-19 and the immunotherapy approaches now helping people with many types of cancer.
Take the case of the latest Nobels. Fundamental questions about how the human body responds to medicinal plants were the initial inspiration behind the work of UCSF’s Julius. He’d noticed that studies from Hungary found that a natural chemical in chili peppers, called capsaicin, activated a subgroup of neurons to create the painful, burning sensation that most of us have encountered from having a bit too much hot sauce. But what wasn’t known was the molecular mechanism by which capsaicin triggered that sensation.
In 1997, having settled on the best experimental approach to study this question, Julius and colleagues screened millions of DNA fragments corresponding to genes expressed in the sensory neurons that were known to interact with capsaicin. In a matter of weeks, they had pinpointed the gene encoding the protein receptor through which capsaicin interacts with those neurons [1]. Julius and team then determined in follow-up studies that the receptor, later named TRPV1, also acts as a thermal sensor on certain neurons in the peripheral nervous system. When capsaicin raises the temperature to a painful range, the receptor opens a pore-like ion channel in the neuron that then transmit a signal for the unpleasant sensation on to the brain.
In collaboration with Patapoutian, Julius then turned his attention from hot to cold. The two used the chilling sensation of the active chemical in mint, menthol, to identify a protein called TRPM8, the first receptor that senses cold [2, 3]. Additional pore-like channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
Taken together, these breakthrough discoveries have opened the door for researchers around the world to study in greater detail how our nervous system detects the often-painful stimuli of hot and cold. Such information may well prove valuable in the ongoing quest to develop new, non-addictive treatments for pain. The NIH is actively pursuing some of those avenues through its Helping to End Addiction Long-termSM (HEAL) Initiative.
Meanwhile, Patapoutian was busy cracking the molecular basis of another basic sense: touch. First, Patapoutian and his collaborators identified a mouse cell line that produced a measurable electric signal when individual cells were poked. They had a hunch that the electrical signal was generated by a protein receptor that was activated by physical pressure, but they still had to identify the receptor and the gene that coded for it. The team screened 71 candidate genes with no luck. Then, on their 72nd try, they identified a touch receptor-coding gene, which they named Piezo1, after the Greek word for pressure [4].
Patapoutian’s group has since found other Piezo receptors. As often happens in basic research, their findings have taken them in directions they never imagined. For example, they have discovered that Piezo receptors are involved in controlling blood pressure and sensing whether the bladder is full. Fascinatingly, these receptors also seem to play a role in controlling iron levels in red blood cells, as well as controlling the actions of certain white blood cells, called macrophages.
Turning now to the 2021 Nobel in Chemistry, the basic research of MacMillan and List has paved the way for addressing a major unmet need in science and industry: the need for less expensive and more environmentally friendly catalysts. And just what is a catalyst? To build the synthetic molecules used in drugs and a wide range of other materials, chemists rely on catalysts, which are substances that control and accelerate chemical reactions without becoming part of the final product.
It was long thought there were only two major categories of catalysts for organic synthesis: metals and enzymes. But enzymes are large, complex proteins that are hard to scale to industrial processes. And metal catalysts have the potential to be toxic to workers, as well as harmful to the environment. Then, about 20 years ago, List and MacMillan, working independently from each other, created a third type of catalyst. This approach, known as asymmetric organocatalysis [5, 6], builds upon small organic molecule catalysts that have a stable framework of carbon atoms, to which more active chemical groups can attach, often including oxygen, nitrogen, sulfur, or phosphorus.
Organocatalysts have gone on to be applied in ways that have proven to be more cost effective and environmentally friendly than using traditional metal or enzyme catalysts. In fact, this precise new tool for molecular construction is now being used to build everything from new pharmaceuticals to light-absorbing molecules used in solar cells.
That brings us to the Nobel Prize in the Economic Sciences. This year’s laureates showed that it’s possible to reach cause-and-effect answers to questions in the social sciences. The key is to evaluate situations in groups of people being treated differently, much like the design of clinical trials in medicine. Using this “natural experiment” approach in the early 1990s, David Card produced novel economic analyses, showing an increase in the minimum wage does not necessarily lead to fewer jobs. In the mid-1990s, Angrist and Imbens then refined the methodology of this approach, showing that precise conclusions can be drawn from natural experiments that establish cause and effect.
Last year, NIH added the names of three scientists to its illustrious roster of Nobel laureates. This year, five more names have been added. Many more will undoubtedly be added in the years and decades ahead. As I’ve said many times over the past 12 years, it’s an extraordinary time to be a biomedical researcher. As I prepare to step down as the Director of this amazing institution, I can assure you that NIH’s future has never been brighter.
References:
[1] The capsaicin receptor: a heat-activated ion channel in the pain pathway. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. Nature 1997:389:816-824.
[2] Identification of a cold receptor reveals a general role for TRP channels in thermosensation. McKemy DD, Neuhausser WM, Julius D. Nature 2002:416:52-58.
[3] A TRP channel that senses cold stimuli and menthol. Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. Cell 2002:108:705-715.
[4] Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Science 2010:330: 55-60.
[5] Proline-catalyzed direct asymmetric aldol reactions. List B, Lerner RA, Barbas CF. J. Am. Chem. Soc. 122, 2395–2396 (2000).
[6] New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels-AlderReaction. Ahrendt KA, Borths JC, MacMillan DW. J. Am. Chem. Soc. 2000, 122, 4243-4244.
Links:
Basic Research – Digital Media Kit (NIH)
Curiosity Creates Cures: The Value and Impact of Basic Research (National Institute of General Medical Sciences/NIH)
Explaining How Research Works (NIH)
NIH Basics, Collins FS, Science, 3 Aug 2012. 337; 6094: 503.
NIH’s Commitment to Basic Science, Mike Lauer, Open Mike Blog, March 25, 2016
Nobel Laureates (NIH)
The Nobel Prize in Physiology or Medicine 2021 (The Nobel Assembly at the Karolinska Institutet, Stockholm, Sweden)
Video: Announcement of the 2021 Nobel Prize in Physiology or Medicine (YouTube)
The Nobel Prize in Chemistry 2021 (The Nobel Assembly at the Karolinska Institutet)
Video: Announcement of the 2021 Nobel Prize in Chemistry (YouTube)
The Nobel Prize in Economic Sciences (The Nobel Assembly at the Karolinska Institutet)
Video: Announcement of the 2021 Nobel Prize in Economic Sciences (YouTube)
Julius Lab (University of California San Francisco)
The Patapoutian Lab (Scripps Research, La Jolla, CA)
Benjamin List (Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany)
The MacMillan Group (Princeton University, NJ)
David Card (University of California, Berkeley)
Joshua Angrist (Massachusetts Institute of Technology, Cambridge)
NIH Support:
David Julius: National Institute of Neurological Diseases and Stroke; National Institute of General Medical Sciences; National Institute of Dental and Craniofacial Research
Ardem Patapoutian: National Institute of Neurological Diseases and Stroke; National Institute of Dental and Craniofacial Research; National Heart, Lung, and Blood Institute
David W.C. MacMillan: National Institute of General Medical Sciences
David Card: National Institute on Aging; Eunice Kennedy Shriver National Institute of Child Health and Human Development
Joshua Angrist: Eunice Kennedy Shriver National Institute of Child Health and Human Development
Next Page
