Finding Better Ways to Image the Retina
Posted on by Dr. Francis Collins
Every day, all around the world, eye care professionals are busy performing dilated eye exams. By looking through a patient’s widened pupil, they can view the retina—the postage stamp-sized tissue lining the back of the inner eye—and look for irregularities that may signal the development of vision loss.
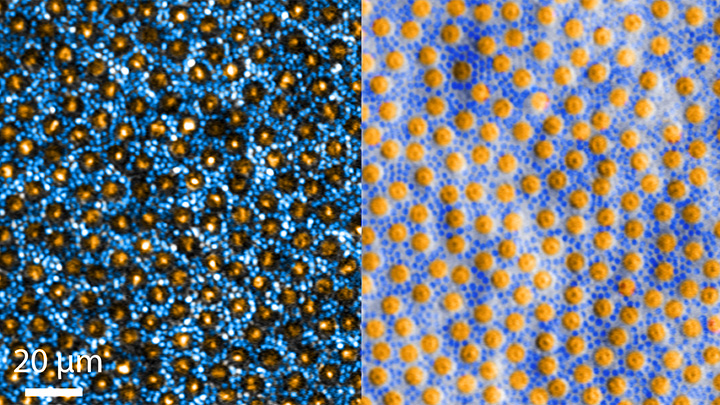
The great news is that, thanks to research, retinal imaging just keeps getting better and better. The images above, which show the same cells viewed with two different microscopic techniques, provide good examples of how tweaking existing approaches can significantly improve our ability to visualize the retina’s two types of light-sensitive neurons: rod and cone cells.
Specifically, these images show an area of the outer retina, which is the part of the tissue that’s observed during a dilated eye exam. Thanks to colorization and other techniques, a viewer can readily distinguish between the light-sensing, color-detecting cone cells (orange) and the much smaller, lowlight-sensing rod cells (blue).
These high-res images come from Johnny Tam, a researcher with NIH’s National Eye Institute. Working with Alfredo Dubra, Stanford University, Palo Alto, CA, Tam and his team figured out how to limit light distortion of the rod cells. The key was illuminating the eye using less light, provided as a halo instead of the usual solid, circular beam.
But the researchers’ solution hit a temporary snag when the halo reflected from the rods and cones created another undesirable ring of light. To block it out, Tam’s team introduced a tiny pinhole, called a sub-Airy disk. Along with use of adaptive optics technology [1] to correct for other distortions of light, the scientists were excited to see such a clear view of individual rods and cones. They published their findings recently in the journal Optica [2]
The resolution produced using these techniques is so much improved (33 percent better than with current methods) that it’s even possible to visualize the tiny inner segments of both rods and cones. In the cones, for example, these inner segments help direct light coming into the eye to other, photosensitive parts that absorb single photons of light. The light is then converted into electrical signals that stream to the brain’s visual centers in the occipital cortex, which makes it possible for us to experience vision.
Tam and team are currently working with physician-scientists in the NIH Clinical Center to image the retinas of people with a variety of retinal diseases, including age-related macular degeneration (AMD), a leading cause of vision loss in older adults. These research studies are ongoing, but offer hopeful possibilities for safe and non-intrusive monitoring of individual rods and cones over time, as well as across disease types. That’s obviously good news for patients. Plus it will help scientists understand how a rod or cone cell stops working, as well as more precisely test the effects of gene therapy and other experimental treatments aimed at restoring vision.
References:
[1] Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Dubra A, Sulai Y, Norris JL, Cooper RF, Dubis AM, Williams DR, Carroll J. Biomed Opt Express. 2011 Jul 1;2(7):1864-76.
[1] In-vivo sub-diffraction adaptive optics imaging of photoreceptors in the human eye with annular pupil illumination and sub-Airy detection. Rongwen L, Aguilera N, Liu T, Liu J, Giannini JP, Li J, Bower AJ, Dubra A, Tam J. Optica 2021 8, 333-343. https://doi.org/10.1364/OPTICA.414206
Links:
Get a Dilated Eye Exam (National Eye Institute/NIH)
How the Eyes Work (NEI)
Eye Health Data and Statistics (NEI)
Tam Lab (NEI)
Dubra Lab (Stanford University, Palo Alto, CA)
NIH Support: National Eye Institute
Share this:
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Snapshots of Life
Tags: adaptive optics techology, age-related macular degeneration, AMD, cone cells, cones, confocal adaptive optics scanning, dilated eye exam, eye, imaging, neurons, occipital cortex, photoreceptor cells, retina, retinal diseases, retinal imaging, rod cell, rods, sub-Airy disk, vision, vision loss, visual cortex

Retinal pigment epithelium and visual acuity is actually a good harbinger of oxidative stress. Surprising large data base with Navy divers (some of which is shared with DAN and the recreational dive community), the Civil Aviation Medical Institute and NASA.
Now is there really a definite answer as to when ATP can be rate limiting in a cell? Perhaps studying senescence in salmon as they migrate upstream to spawn can be revealing?
Monoamine transporters and their role in myopia based on DA packaging has been studied. In countries that maintain longitudinal health data on it’s people, it’s rather interesting. Particularly Northern Ireland and statistically higher prescription of certain medication compared to other territories serviced by NHS.