Replenishing the Liver’s Immune Protections
Posted on by Dr. Francis Collins
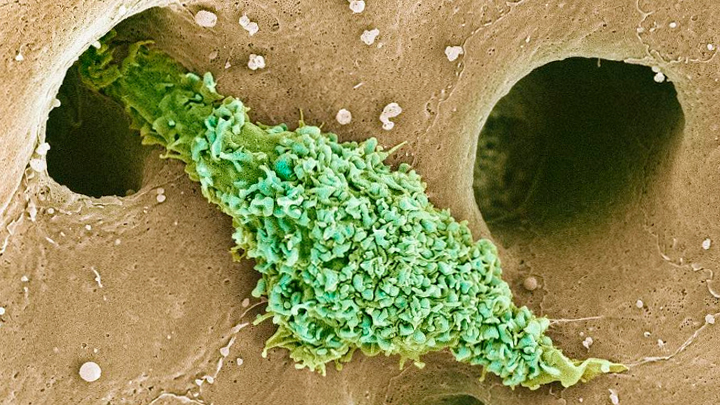
Most of our immune cells circulate throughout the bloodstream to serve as a roving security force against infection. But some immune cells don’t travel much at all and instead safeguard a specific organ or tissue. That’s what you are seeing in this electron micrograph of a type of scavenging macrophage, called a Kupffer cell (green), which resides exclusively in the liver (brown).
Normally, Kupffer cells appear in the liver during the early stages of mammalian development and stay put throughout life to protect liver cells, clean up old red blood cells, and regulate iron levels. But in their experimental system, Christopher Glass and his colleagues from University of California, San Diego, removed all original Kupffer cells from a young mouse to see if this would allow signals from the liver that encourage the development of new Kupffer cells.
The NIH-funded researchers succeeded in setting up the right conditions to spur a heavy influx of circulating precursor immune cells, called monocytes, into the liver, and then prompted those monocytes to turn into the replacement Kupffer cells. In a recent study in the journal Immunity, the team details the specific genomic changes required for the monocytes to differentiate into Kupffer cells [1]. This information will help advance the study of Kupffer cells and their role in many liver diseases, including nonalcoholic steatohepatitis (NASH), which affects an estimated 3 to 12 percent of U.S. adults [2].
The new work also has broad implications for immunology research because it provides additional evidence that circulating monocytes contain genomic instructions that, when activated in the right way by nearby cells or other factors, can prompt the monocytes to develop into various, specialized types of scavenging macrophages. For example, in the mouse system, Glass’s team found that the endothelial cells lining the liver’s blood vessels, which is where Kupffer cells hang out, emit biochemical distress signals when their immune neighbors disappear.
While more details need to be worked out, this study is another excellent example of how basic research, including the ability to query single cells about their gene expression programs, is generating fundamental knowledge about the nature and behavior of living systems. Such knowledge is opening new possibilities to more precise ways of treating and preventing diseases all throughout the body, including those involving Kupffer cells and the liver.
References:
[1] Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, Ego KM, Bruni CM, Deng Z, Schlachetzki JCM, Nott A, Bennett H, Chang J, Vu BT, Pasillas MP, Link VM, Texari L, Heinz S, Thompson BM, McDonald JG, Geissmann F3, Glass CK. Immunity. 2019 Oct 15;51(4):655-670.
[2] Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Spengler EK, Loomba R. Mayo Clinic Proceedings. 2015;90(9):1233–1246.
Links:
Liver Disease (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Nonalcoholic Fatty Liver Disease & NASH (NIDDK)
Glass Laboratory (University of California, San Diego)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of General Medical Sciences; National Cancer Institute

A thought-provoking innovative scientific article highlighting liver-immunobiology with promising immunotherapeutic and translational research public health oriented impact!
NASH, NAFLD, liver cirrhosis, Alcoholic hepatic steatosis and associated co-morbidities are emerging as leading public health challenges worldwide; aberrant metabolic/biochemical signaling networks with regenerative medicine/stem cells appears an attractive strategy in critically analysing heterogeneous cell-lineages in ethnically disparate asymptomatic/normal vs borderline vs diseased/symptomatic population-subsets of varying lifestyles and ethnic profiles.
An elegant article with long-term clinical impact dissecting immunomodulation/immunosurveillance in the complex liver physiology.
Excellent post. However, what could be a simple matter of personal preference, I would always keep for the information in the genome words that highlight their passive, library-like function that is read and used by cells to adapt to a new condition. In this case, the cells of the enothelium, realizing the absence of the experimentally removed Kupffer cells, use their information to respond to this condition by sending biochemical signals so that the monocytes also use their library of genetic information and become new Kupfer cells. . Whenever the genome is actively presented as the “doing things” component of cells, the bad custom of determinism and its remarkable association with lazy minds is reinforced.
Hey guys im so happy to see this advances on this type of diseases, my question is: will this make a better healthy life for a person living with G6PD deficiency, because I have the condition??
Thank you author. Keep it up.
Nice post author. Thank you.
What a nice post. Thank you.
Nice post.Thank you.
Nice post. Thank you.
The information was very helpful. Keep writing such informative articles, It help us to understand many things. Good work. Keep writing.
The information that you provided is very helpful. It is very descriptive and helped me to understand the whole scenario.