MicroED: From Powder to Structure in a Half-Hour
Posted on by Dr. Francis Collins
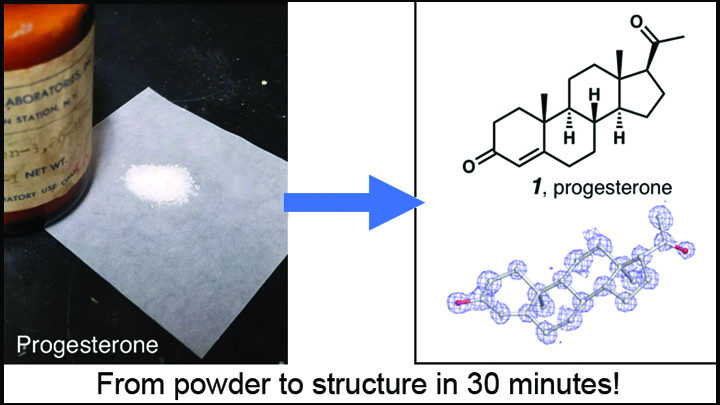
Credit: Adapted from Jones et al. ChemRxiv.org
Over the past few years, there’s been a great deal of excitement about the power of cryo-electron microscopy (cryo-EM) for mapping the structures of large biological molecules like proteins and nucleic acids. Now comes word of another absolutely incredible use of cryo-EM: determining with great ease and exquisite precision the structure of the smaller organic chemical compounds, or “small molecules,” that play such key roles in biological exploration and drug development.
The new advance involves a cryo-EM technique called microcrystal-electron diffraction (MicroED). As detailed in a preprint on ChemRxiv.org [1] and the journal Angewandte Chemie [2], MicroED has enabled researchers to take the powdered form of commercially available small molecules and generate high-resolution data on their chemical structures in less than a half-hour—dramatically faster than with traditional methods!
Now, for a bit of context: X-ray crystallography has long been the mainstay for determining the structure of molecules, both large and small. The method involves growing molecules in crystal form, firing X-rays at those crystals, and then analyzing the X-ray diffraction patterns to infer a molecular structure. The catch is that X-ray crystallography requires large crystals, which are simply impossible to produce for many molecules. Another common structural mapping technology, called nuclear magnetic resonance (NMR) spectroscopy, only provides indirect information about molecular structure and can’t deal with heterogeneous mixtures.
That’s where the excitement about cryo-EM comes in. The technique does not involve the slow, complicated process of growing large crystals in a lab container. Instead, it images single nano-crystals that are flash frozen, bombarding them with electrons to capture their images with a special camera.
MicroED, the cryo-EM approach generating so much excitement in this report, was first introduced in 2013 by Tamir Gonen, an investigator at the Howard Hughes Medical Institute, who is now at the University of California, Los Angeles (UCLA) [3]. The approach takes advantage of cryo-electron microscopes’ ability to perform diffraction experiments on crystals in a manner similar to X-ray crystallography, but with a new twist: much smaller crystals can be used.
Specifically, MicroED involves shooting electrons at tiny, flash-frozen crystals and collecting the resulting diffraction patterns. Because electrons interact with matter more strongly than X-rays, it’s possible to obtain useful data from very tiny crystals. In fact, MicroED makes it possible to generate atomic-resolution structures from crystals that are a billion times smaller in weight than those needed for X-ray crystallography!
While MicroED’s use for determining the structure of larger molecules like proteins remains challenging, the new study demonstrates its remarkable potential for analyzing small molecules, which include most of our current medicines. In their first test of MicroED on small molecules, Gonen and his California colleagues including Hosea Nelson, Jose Rodriguez, and Brian Stolz took some commercially available progesterone powder straight from a 20-year-old bottle. To their delight, thousands of tiny crystals were already present in the powder, and they were able to determine the structure of the small molecule down to the atomic level in less than a half-hour! In separate experiments, the same concept was also demonstrated by a Swiss/German collaboration, led by Tim Grüne at the Paul Scherrer Institute, Villigen, Switzerland.
Emboldened by their initial findings, Gonen’s team decided to use MicroED to analyze samples of a variety of other small molecules, including acetaminophen and ibuprofen, medicines taken right off store shelves, and the prescription anticonvulsant drug carbamazepine. Also analyzed were eight less-familiar small molecules. All told, nearly every sample they tried yielded a structure with ease.
The researchers then went on to mix four small-molecule compounds together, and MicroED had no problem in determining the structures of all within minutes. What’s more, they’ve been able to resolve the structures of compounds with crystals that are virtually invisible—too small to be seen, even under a light microscope.
With this latest advance, it appears that MicroED may prove to be a powerful structural-mapping tool for researchers trying to design small molecule probes to study various disease processes or searching for candidate small molecules to treat or prevent disease. Because the approach relies on a lower-cost version of the same equipment and cameras as any other cryo-EM method, existing facilities, including the three NIH-funded, national cryo-EM service centers [4], already are equipped for MicroED.
This is also a good moment to highlight the fact that more and more scientific papers, including the one featured here, are now appearing first in preprint servers. That’s a positive thing in terms of providing the scientific community with early access to exciting new developments. It’s important to keep in mind, however, that preprint findings have not necessarily undergone rigorous peer review by other scientists, so it’s always possible a few things might change after that process is completed. In this case, Gonen reports that this paper has now been peer-reviewed and accepted for publication by ACS Central Science.
References:
[1] The cryoEM method MicroED as a powerful tool for small molecule structure determination. Jones CG, Martynowycz MW, Hattne J, Fulton T, Stoltz BM, Rodriguez JA, Nelson HM, Gonen T. ChemRxiv. October 17, 2018.
[2] Rapid structure determination of microcrystalline molecular compounds using electron diffraction. Gruene T, Wennmacher JTC, Zaubitzer C, Holstein JJ, Heidler J, Fecteau-Lefebvre A, De Carlo S, Müller E, Goldie KN, Regeni I10, Li T, Santiso-Quinones G, Steinfeld G, Handschin S, van Genderen E, van Bokhoven JA, Clever GH, Pantelic R. Angew Chem Int Ed Engl. 2018 Oct 16. [Epub ahead of print]
[3] Three-dimensional electron crystallography of protein microcrystals. Shi D, Nannenga BL, Iadanza MG, Gonen T. Elife. 2013 Nov 19;2:e01345.
[4] NIH funds three national cryo-EM service centers and training for new microscopists. National Institutes of Health. May 15, 2018.
Links:
Micro-Electron Diffraction (Howard Hughes Medical Institute, Janelia Campus, Ashburn, VA)
Gonen Lab (University of California, Los Angeles)
Tamir Gonen (Howard Hughes Medical Institute)
NIH Support: National Institute of General Medical Sciences

It’s unfortunate that the paper of the Swiss-German collaboration who published the same method prior to the preprint is not mentioned at all in this blog entry. Or is this omission on purpose?
No, it was an accidental omission. I apologize for not mentioning the preprint in the original blog post, and the paper is now noted in the text.
Paper of Tamir is in ACS Central Science now with “POST PREPRINT ADDENDUM AND BACKGROUND”, at-least which is better than claiming that this is a new discovery as claimed by the Authors in different science magazines. In the current version of the paper, also the reference number 24 “Sub-ångström cryo-EM structure of a prion protofibril reveals a polar clasp” talks about carbamazepine structure by so called MicroED, Same carbamazepine structure has been solved at atomic resolution by direct methods by Electron Diffraction (ED) with continuous rotation method by van Genderen et al. in 2015 and published. Also there is another paper on structure solution of 2 organic compound by ED came online on Sep, 2018 (https://pubs.acs.org/doi/10.1021/acs.oprd.8b00149). Is these omissions are done on purpose for the future funding?
This is really exciting. MicroED just keeps getting better and better.
Thanks for sharing a great info and keep sharing with us. Progesterone is a crucial part of the menstrual cycle and maintenance of pregnancy. Progesterone helps to regulate your cycle.