Brain Imaging: Advance Aims for Epilepsy’s Hidden Hot Spots
Posted on by Dr. Francis Collins
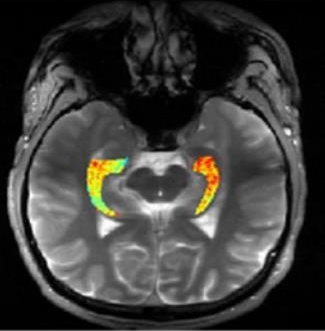
Credit: Reddy Lab, University of Pennsylvania
For many of the 65 million people around the world with epilepsy, modern medications are able to keep the seizures under control. When medications fail, as they do in about one-third of people with epilepsy, surgery to remove affected brain tissue without compromising function is a drastic step, but offers a potential cure. Unfortunately, not all drug-resistant patients are good candidates for such surgery for a simple reason: their brains appear normal on traditional MRI scans, making it impossible to locate precisely the source(s) of the seizures.
Now, in a small study published in Science Translational Medicine [1], NIH-funded researchers report progress towards helping such people. Using a new MRI method, called GluCEST, that detects concentrations of the nerve-signaling chemical glutamate in brain tissue [2], researchers successfully pinpointed seizure-causing areas of the brain in four of four volunteers with drug-resistant epilepsy and normal traditional MRI scans. While the findings are preliminary and must be confirmed by larger studies, researchers are hopeful that GluCEST, which takes about 30 minutes, may open the door to new ways of treating this type of epilepsy.
To understand why glutamate might be a useful marker for epilepsy, it’s useful to note that this molecule serves as the brain’s main excitatory nerve signal. In healthy people, when neurons fire off a stimulatory signal, they release short bursts of glutamate to relay the signal onward. Support cells then rapidly clear the glutamate. But in some people with epilepsy, glutamate seems to build up outside of brain cells, triggering continuous overstimulation and potential seizures.
The challenge has been finding these suspected glutamate hot spots, to see if they correlate with seizures. In the study, led by Ravinder Reddy and Kathryn Davis of the University of Pennsylvania, the researchers turned to GluCEST to image hot spots specifically in people with drug-resistant epilepsy localized to the brain’s temporal lobe. This includes the left and right hippocampi, which are parts of the brain that help to consolidate short-term thought into long-term memory, play a role in spatial navigation, and, importantly, are a frequent site of seizures.
While conventional MRI works by using strong magnets and radio waves to study water molecules directly in living tissue, GluCEST takes advantage of special chemical properties of glutamate that influence water to visualize and quantify it at high resolution. Reddy and Davis found in the study that their GluCEST images could detect an increase in glutamate in the hippocampi. In every case, glutamate levels were elevated on the side of the brain in which the participant’s seizures were found to originate, based on brain wave recordings during a seizure. In contrast to the people with epilepsy, GluCEST scans of 11 healthy volunteers didn’t show any consistent differences in glutamate levels on one side of the brain versus the other.
Although the new study represents a very small number of patients, it suggests that GluCEST might stand to improve patient care and quality of life in people with epilepsy who now have very limited treatment options. The researchers say they’ve reported their early findings in hopes that they can be validated in more people with epilepsy by researchers at other medical centers. The GluCEST technique requires powerful 7 Tesla MRI scanners, which are already in regular use for research purposes at some medical centers around the country, and special software, which the researchers intend to make freely available.
As for the UPenn team, they’re working to expand and improve upon the GluCEST method to produce images and data in three dimensions and over a wider swath of the brain. If they are successful, this new tool could yield important insights into epilepsy and a variety of other neurological disorders in which glutamate is thought to play an important role, including schizophrenia, Alzheimer’s disease, Parkinson’s disease, and maybe even autism.
References:
[1] Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Davis KA, Nanga RP, Das S, Chen SH, Hadar PN, Pollard JR, Lucas TH, Shinohara RT, Litt B, Hariharan H, Elliott MA, Detre JA, Reddy R. Sci Transl Med. 2015 Oct 14;7(309):309ra161.
[2] Magnetic resonance imaging of glutamate. Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Nat Med. 2012 Jan 22;18(2):302-306.
Links:
Epilepsy Information Page (National Institute of Neurological Disorders and Stroke/NIH)
Magnetic Resonance Imaging (National Institute of Biomedical Imaging and Bioengineering/NIH)
Center For Magnetic Resonance And Optical Imaging (Perelman School of Medicine, University of Pennsylvania, Philadelphia)
Kathryn Davis (Perelman School of Medicine)
Ravinder Reddy (Perelman School of Medicine)
NIH Support: National Institute of Biomedical Imaging and Bioengineering; National Institute of Neurological Disorders and Stroke
