Prostate Cancer: Designing a Smarter High-Tech Biopsy
Posted on by Dr. Francis Collins
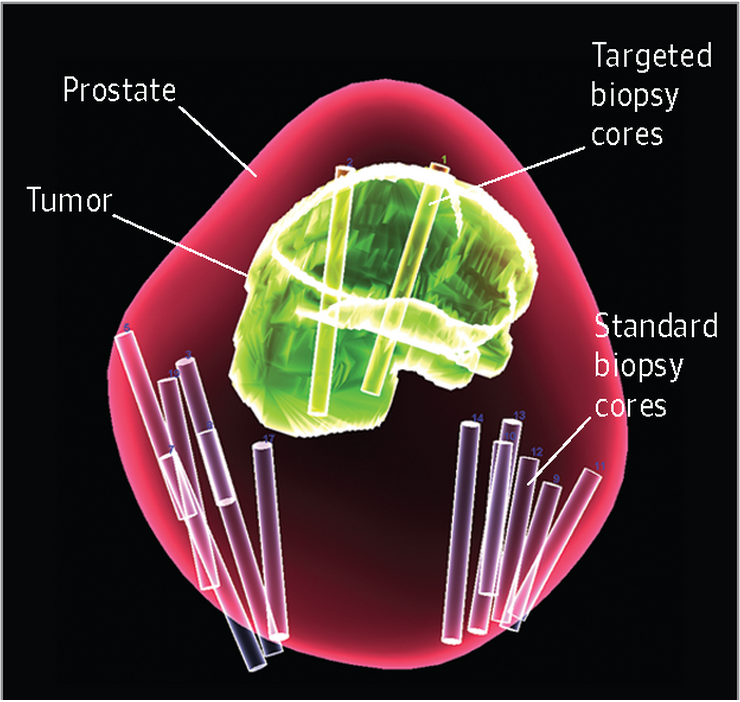
Caption: When the biopsy is completed, a 3D data map is generated. In this actual example, what is shown is the contour of the prostate (red), location of the tumor (green), the location of the standard random prostate biopsies (white cores), and the location of the targeted fusion biopsies (yellow cores).
Credit: Peter A. Pinto, National Cancer Institute, NIH
Many of you probably know that prostate cancer is the most frequently diagnosed cancer in American men. But here’s something that might surprise you: the way in which doctors biopsy for prostate cancer hasn’t changed significantly in nearly 30 years—even though about a million such biopsies are conducted every year in the United States.
Unlike breast cancer biopsies, which sample tissue from a suspicious area seen on a mammogram, prostate cancer biopsies still are generally performed as random, 12-point searches to see if any cancerous cells might be lurking somewhere in the prostate gland. While random biopsies have helped to save many lives, NIH-supported research has developed a targeted approach that brings much-needed efficiency to the diagnostic process—and appears to be better at detecting aggressive, high-risk prostate cancer than current methods.
The approach, called “targeted fusion biopsy,” was envisioned nearly 10 years ago by a team of intramural researchers at the NIH Clinical Center in Bethesda, MD. Under a cooperative research and development agreement with the Florida-based Philips Healthcare, a prototype imaging system called UroNav was created, tested, and recently approved by the Food and Drug Administration. And, now, researchers report that fusion biopsy has produced impressive results in a seven-year clinical trial involving more than 1,000 men [1].
Before we dive into the study’s results, here’s a quick primer on targeted fusion biopsy technology. The secret lies in a sophisticated software package, capable of running on an ordinary laptop computer, that fuses images produced by two technologies often used in detecting prostate cancer—ultrasound and magnetic resonance imaging (MRI)—and allows them to communicate for the first time.
And here’s how the diagnostic process works from the patient perspective. A man first undergoes MRI to generate a high-resolution snapshot of his prostate. A radiologist reads the MRI like a mammogram, scoring any visual abnormalities as low, intermediate, or high grade, with the latter as the most worrisome. If a potentially serious abnormality is seen in the MRI, the patient is called back for a biopsy. His MRI is uploaded to the laptop, reconstructed into a 3D image, and superimposed like an anatomical map over a 3D real-time ultrasound monitor on the laptop.
Now, it’s time for the biopsy. Attached to the ultrasound probe in the rectum is an electromagnetic sensor that serves as a red-dot tracker on the laptop to show the probe’s exact 3D location within the prostate. When the probe reaches the problem area, as shown on the computer screen, a sample or two of prostate tissue is surgically removed and sent to a pathologist for further analysis.
In findings just published in the journal JAMA, a National Cancer Institute (NCI) team, led by surgeon Peter Pinto, reported results of the first head-to-head comparison of the targeted fusion approach and standard, random biopsy to see which was better at detecting clinically meaningful prostate cancer. Pinto and his colleagues wanted to know whether fusion biopsy could stand on its own without the help of random biopsy as a backup to catch clinically important tumors that it might overlook.
The prospective study involved 1,003 men with an abnormal digital rectal exam and/or elevated blood serum levels of prostate-specific antigen (PSA), both which can be associated with prostate cancer. Once enrolled, patients received a targeted fusion biopsy performed by a team physician. Later, each man received a standard, 12-point random biopsy conducted by another team member, who was blinded to the results of the targeted biopsy.
The researchers found the targeted fusion biopsy more than held its own against standard biopsy, detecting 30 percent more aggressive prostate cancers than the random biopsy. These are the type of high-risk tumors that evidence suggests need to be detected with greater frequency to save lives. Interestingly, fusion biopsy detected 17 percent fewer low-risk cancers than the random biopsy because the MRI literally didn’t see them. This shortfall isn’t necessarily a bad thing because such slow-growing cancers often do not threaten the patient’s health. Like a mole on the skin, removing them might never be medically necessary. In fact, oncologists have debated for years whether performing surgery to remove slow-growing tumors might, in some instances, constitute overtreatment that does more harm than good to the patient and his quality of life.
In the second part of the study, the NCI team explored the question of whether targeted fusion biopsy could better guide a decision about whether to recommend surgery to remove the prostate, or to watch and wait. To get at this issue, researchers studied a subset of study participants who actually underwent surgery to remove their prostate. The team’s pathologists analyzed these prostate glands by standard microscopy to see how that information stacked up against that gathered by MRI imaging. They found that MRI and subsequent targeted biopsy provided a more accurate assessment of the aggressiveness of a prostate cancer than standard, random biopsy. If these findings are confirmed in follow-up studies, it suggests that targeted fusion biopsies may prove helpful in the process of deciding whether to pursue surgery for prostate cancer or opt for a “watchful waiting” approach.
The next step will be to determine whether the fusion biopsy approach actually improves men’s long-term survival, by allowing aggressive treatment of those prostate cancers that are most likely to spread to other parts of the body, and by limiting interventions for cancers that are slow-growing.
Since the development of UroNav, five other targeted fusion biopsy platforms have been developed and commercialized in Europe, Asia, and North America. So no doubt we will be hearing a lot more about targeted fusion biopsies not only for prostate cancer, but other types of cancer as well.
Reference:
- Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Siddiqui M, Rais-Bahrami, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, and Pinto PA. JAMA. 2015 January 27;313(4):390-7.
Links:
Prostate Cancer, National Cancer Institute (NCI/NIH)
Pinto Lab, NCI
Audio: Dr. Pinto discusses targeted fusion biopsy for prostate cancer. JAMA Network
Wood Lab, NIH Clinical Center
NIH Support: National Cancer Institute and NIH Clinical Center

I want to thanks for your time for this wonderful Article!! …